Rdh10 loss-of-function and perturbed retinoid signaling underlies the etiology of choanal atresia
- PMID: 28169399
- PMCID: PMC5390677
- DOI: 10.1093/hmg/ddx031
Rdh10 loss-of-function and perturbed retinoid signaling underlies the etiology of choanal atresia
Abstract
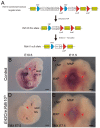
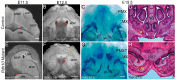
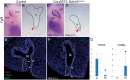
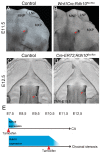
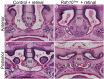
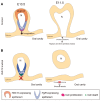
Craniofacial development is a complex process that involves sequential growth and fusion of the facial prominences. When these processes fail, congenital craniofacial anomalies can occur. For example, choanal atresia (CA) is a congenital craniofacial anomaly in which the connection between the nasal airway and nasopharynx is completely blocked. CA occurs in approximately 1/5000 live births and is a frequent component of congenital disorders such as CHARGE, Treacher Collins, Crouzon and Pfeiffer syndromes. However, the detailed cellular and molecular mechanisms underpinning the etiology and pathogenesis of CA remain elusive. In this study, we discovered that mice with mutations in retinol dehydrogenase 10 (Rdh10), which perturbs Vitamin A metabolism and retinoid signaling, exhibit fully penetrant CA. Interestingly, we demonstrate Rdh10 is specifically required in non-neural crest cells prior to E10.5 for proper choanae formation, and that in the absence of Rdh10, Fgf8 is ectopically expressed in the nasal fin. Furthermore, we found that defects in choanae development are associated with decreased cell proliferation and increased cell death in the epithelium of the developing nasal cavity, which retards invagination of the nasal cavity, and thus appears to contribute to the pathogenesis of CA. Taken together, our findings demonstrate that RDH10 is essential during the early stages of facial morphogenesis for the formation of a functional nasal airway, and furthermore establish Rdh10 mutant mice as an important model system to study CA.
© The Author 2017. Published by Oxford University Press.
Figures

Similar articles
-
Choanal Atresia and Craniosynostosis: Development and Disease.Plast Reconstr Surg. 2018 Jan;141(1):156-168. doi: 10.1097/PRS.0000000000003928. Plast Reconstr Surg. 2018. PMID: 29280877 Free PMC article. Review.
-
Choanal atresia and stenosis: Development and diseases of the nasal cavity.Wiley Interdiscip Rev Dev Biol. 2019 Jan;8(1):e336. doi: 10.1002/wdev.336. Epub 2018 Oct 15. Wiley Interdiscip Rev Dev Biol. 2019. PMID: 30320458 Review.
-
Synergistic role of retinoic acid signaling and Gata3 during primitive choanae formation.Hum Mol Genet. 2021 Nov 30;30(24):2383-2392. doi: 10.1093/hmg/ddab205. Hum Mol Genet. 2021. PMID: 34272563
-
Quantification of shape and cell polarity reveals a novel mechanism underlying malformations resulting from related FGF mutations during facial morphogenesis.Hum Mol Genet. 2013 Dec 20;22(25):5160-72. doi: 10.1093/hmg/ddt369. Epub 2013 Aug 1. Hum Mol Genet. 2013. PMID: 23906837 Free PMC article.
-
RDH10 oxidation of Vitamin A is a critical control step in synthesis of retinoic acid during mouse embryogenesis.PLoS One. 2012;7(2):e30698. doi: 10.1371/journal.pone.0030698. Epub 2012 Feb 2. PLoS One. 2012. PMID: 22319578 Free PMC article.
Cited by
-
Mechanisms of Feedback Regulation of Vitamin A Metabolism.Nutrients. 2022 Mar 21;14(6):1312. doi: 10.3390/nu14061312. Nutrients. 2022. PMID: 35334970 Free PMC article. Review.
-
Jak2 and Jaw Muscles Are Required for Buccopharyngeal Membrane Perforation during Mouth Development.J Dev Biol. 2023 May 31;11(2):24. doi: 10.3390/jdb11020024. J Dev Biol. 2023. PMID: 37367478 Free PMC article.
-
Choanal Atresia and Craniosynostosis: Development and Disease.Plast Reconstr Surg. 2018 Jan;141(1):156-168. doi: 10.1097/PRS.0000000000003928. Plast Reconstr Surg. 2018. PMID: 29280877 Free PMC article. Review.
-
Genetically programmed retinoic acid deficiency during gastrulation phenocopies most known developmental defects due to acute prenatal alcohol exposure in FASD.Front Cell Dev Biol. 2023 Jun 16;11:1208279. doi: 10.3389/fcell.2023.1208279. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37397253 Free PMC article.
-
RDH10-mediated retinol metabolism and RARα-mediated retinoic acid signaling are required for submandibular salivary gland initiation.Development. 2018 Aug 2;145(15):dev164822. doi: 10.1242/dev.164822. Development. 2018. PMID: 29986869 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

