Understanding the Molecular Basis of Multiple Mitochondrial Dysfunctions Syndrome 1 (MMDS1)-Impact of a Disease-Causing Gly208Cys Substitution on Structure and Activity of NFU1 in the Fe/S Cluster Biosynthetic Pathway
- PMID: 28161430
- PMCID: PMC5466808
- DOI: 10.1016/j.jmb.2017.01.021
Understanding the Molecular Basis of Multiple Mitochondrial Dysfunctions Syndrome 1 (MMDS1)-Impact of a Disease-Causing Gly208Cys Substitution on Structure and Activity of NFU1 in the Fe/S Cluster Biosynthetic Pathway
Abstract
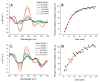
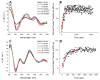
Iron-sulfur (Fe/S)-cluster-containing proteins constitute one of the largest protein classes, with varied functions that include electron transport, regulation of gene expression, substrate binding and activation, and radical generation. Consequently, the biosynthetic machinery for Fe/S clusters is evolutionarily conserved, and mutations in a variety of putative intermediate Fe/S cluster scaffold proteins can cause disease states, including multiple mitochondrial dysfunctions syndrome (MMDS), sideroblastic anemia, and mitochondrial encephalomyopathy. Herein, we have characterized the impact of defects occurring in the MMDS1 disease state that result from a point mutation (Gly208Cys) near the active site of NFU1, an Fe/S scaffold protein, via an in vitro investigation into the structural and functional consequences. Analysis of protein stability and oligomeric state demonstrates that the mutant increases the propensity to dimerize and perturbs the secondary structure composition. These changes appear to underlie the severely decreased ability of mutant NFU1 to accept an Fe/S cluster from physiologically relevant sources. Therefore, the point mutation on NFU1 impairs downstream cluster trafficking and results in the disease phenotype, because there does not appear to be an alternative in vivo reconstitution path, most likely due to greater protein oligomerization from a minor structural change.
Keywords: NFU1; cluster exchange; iron–sulfur cluster; mitochondrial disease; protein stability.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures

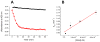


Similar articles
-
Understanding the molecular basis for multiple mitochondrial dysfunctions syndrome 1 (MMDS1): impact of a disease-causing Gly189Arg substitution on NFU1.FEBS J. 2017 Nov;284(22):3838-3848. doi: 10.1111/febs.14271. Epub 2017 Oct 12. FEBS J. 2017. PMID: 28906594 Free PMC article.
-
Analysis of NFU-1 metallocofactor binding-site substitutions-impacts on iron-sulfur cluster coordination and protein structure and function.FEBS J. 2017 Nov;284(22):3817-3837. doi: 10.1111/febs.14270. Epub 2017 Oct 16. FEBS J. 2017. PMID: 28906593
-
Assembly of the [4Fe-4S] cluster of NFU1 requires the coordinated donation of two [2Fe-2S] clusters from the scaffold proteins, ISCU2 and ISCA1.Hum Mol Genet. 2020 Nov 25;29(19):3165-3182. doi: 10.1093/hmg/ddaa172. Hum Mol Genet. 2020. PMID: 32776106 Free PMC article.
-
Steps Toward Understanding Mitochondrial Fe/S Cluster Biogenesis.Methods Enzymol. 2018;599:265-292. doi: 10.1016/bs.mie.2017.09.004. Epub 2017 Nov 27. Methods Enzymol. 2018. PMID: 29746243 Review.
-
Mitochondrial iron-sulfur protein biogenesis and human disease.Biochimie. 2014 May;100:61-77. doi: 10.1016/j.biochi.2014.01.010. Epub 2014 Jan 23. Biochimie. 2014. PMID: 24462711 Review.
Cited by
-
Patient-specific variants of NFU1/NFU-1 disrupt cholinergic signaling in a model of multiple mitochondrial dysfunctions syndrome 1.Dis Model Mech. 2023 Feb 1;16(2):dmm049594. doi: 10.1242/dmm.049594. Epub 2023 Feb 1. Dis Model Mech. 2023. PMID: 36645076 Free PMC article.
-
Privileged Electrophile Sensors: A Resource for Covalent Drug Development.Cell Chem Biol. 2017 Jul 20;24(7):787-800. doi: 10.1016/j.chembiol.2017.05.023. Epub 2017 Jun 22. Cell Chem Biol. 2017. PMID: 28648380 Free PMC article. Review.
-
Reconstitution, characterization, and [2Fe-2S] cluster exchange reactivity of a holo human BOLA3 homodimer.J Biol Inorg Chem. 2019 Oct;24(7):1035-1045. doi: 10.1007/s00775-019-01713-x. Epub 2019 Sep 5. J Biol Inorg Chem. 2019. PMID: 31486956 Free PMC article.
-
Allele-specific mitochondrial stress induced by Multiple Mitochondrial Dysfunctions Syndrome 1 pathogenic mutations modeled in Caenorhabditis elegans.PLoS Genet. 2021 Aug 27;17(8):e1009771. doi: 10.1371/journal.pgen.1009771. eCollection 2021 Aug. PLoS Genet. 2021. PMID: 34449775 Free PMC article.
-
Regulation of human Nfu activity in Fe-S cluster delivery-characterization of the interaction between Nfu and the HSPA9/Hsc20 chaperone complex.FEBS J. 2018 Jan;285(2):391-410. doi: 10.1111/febs.14353. Epub 2017 Dec 29. FEBS J. 2018. PMID: 29211945 Free PMC article.
References
-
- Sreerama N, Woody RW. Estimation of protein secondary structure from CD spectra: Comparison of CONTIN, SELCON and CDSSTR methods with an expanded reference set. Anal Chem. 2000;287:252–60. - PubMed
-
- Kuzmic P. Program DYNAFIT for the analysis of enzyme kinetic data: Application to HIV proteinase. Anal Biochem. 1996;237:260–73. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

