Aurora kinases: novel therapy targets in cancers
- PMID: 28147341
- PMCID: PMC5410356
- DOI: 10.18632/oncotarget.14893
Aurora kinases: novel therapy targets in cancers
Abstract
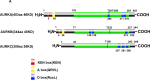
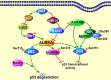
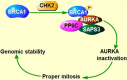
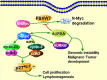
Aurora kinases, a family of serine/threonine kinases, consisting of Aurora A (AURKA), Aurora B (AURKB) and Aurora C (AURKC), are essential kinases for cell division via regulating mitosis especially the process of chromosomal segregation. Besides regulating mitosis, Aurora kinases have been implicated in regulating meiosis. The deletion of Aurora kinases could lead to failure of cell division and impair the embryonic development. Overexpression or gene amplification of Aurora kinases has been clarified in a number of cancers. And a growing number of studies have demonstrated that inhibition of Aurora kinases could potentiate the effect of chemotherapies. For the past decades, a series of Aurora kinases inhibitors (AKIs) developed effectively repress the progression and growth of many cancers both in vivo and in vitro, suggesting that Aurora kinases could be a novel therapeutic target. In this review, we'll first briefly present the structure, localization and physiological functions of Aurora kinases in mitosis, then describe the oncogenic role of Aurora kinases in tumorigenesis, we shall finally discuss the outcomes of AKIs combination with conventional therapy.
Keywords: Aurora kinases; Aurora kinases inhibitors; cancer therapy target; combination therapy; mitosis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Recent Updates on Oncogenic Signaling of Aurora Kinases in Chemosensitive, Chemoresistant Cancers: Novel Medicinal Chemistry Approaches for Targeting Aurora Kinases.Curr Med Chem. 2024;31(23):3502-3528. doi: 10.2174/0929867330666230503124408. Curr Med Chem. 2024. PMID: 37138483 Review.
-
Aurora-A Kinase: A Potent Oncogene and Target for Cancer Therapy.Med Res Rev. 2016 Nov;36(6):1036-1079. doi: 10.1002/med.21399. Epub 2016 Jul 13. Med Res Rev. 2016. PMID: 27406026 Review.
-
Aurora kinases: new molecular targets in thyroid cancer therapy.Clin Ter. 2012 Nov;163(6):e457-62. Clin Ter. 2012. PMID: 23306762 Review.
-
Aurora Kinase Inhibitors in Head and Neck Cancer.Curr Top Med Chem. 2018;18(3):199-213. doi: 10.2174/1568026618666180112163741. Curr Top Med Chem. 2018. PMID: 29332580 Review.
-
Aurora kinases: new targets for cancer therapy.Clin Cancer Res. 2006 Dec 1;12(23):6869-75. doi: 10.1158/1078-0432.CCR-06-1405. Clin Cancer Res. 2006. PMID: 17145803 Review.
Cited by
-
High-throughput kinase inhibitor screening reveals roles for Aurora and Nuak kinases in neurite initiation and dendritic branching.Sci Rep. 2021 Apr 14;11(1):8156. doi: 10.1038/s41598-021-87521-3. Sci Rep. 2021. PMID: 33854138 Free PMC article.
-
Evaluation of the upregulation and surface expression of hypoxanthine guanine phosphoribosyltransferase in acute lymphoblastic leukemia and Burkitt's B cell lymphoma.Cancer Cell Int. 2020 Aug 6;20:375. doi: 10.1186/s12935-020-01457-8. eCollection 2020. Cancer Cell Int. 2020. PMID: 32782434 Free PMC article.
-
Effects of Shenkang Pills on Early-Stage Diabetic Nephropathy in db/db Mice via Inhibiting AURKB/RacGAP1/RhoA Signaling Pathway.Front Pharmacol. 2022 Feb 11;13:781806. doi: 10.3389/fphar.2022.781806. eCollection 2022. Front Pharmacol. 2022. PMID: 35222021 Free PMC article.
-
When Just One Phosphate Is One Too Many: The Multifaceted Interplay between Myc and Kinases.Int J Mol Sci. 2023 Mar 1;24(5):4746. doi: 10.3390/ijms24054746. Int J Mol Sci. 2023. PMID: 36902175 Free PMC article. Review.
-
Analysis of Predicted Amino Acid Sequences of Diatom Microtubule Center Components.Int J Mol Sci. 2023 Aug 14;24(16):12781. doi: 10.3390/ijms241612781. Int J Mol Sci. 2023. PMID: 37628962 Free PMC article.
References
-
- Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol. 2001;2:21–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

