Cholesterol Enrichment Impairs Capacitative Calcium Entry, eNOS Phosphorylation & Shear Stress-Induced NO Production
- PMID: 28138348
- PMCID: PMC5270765
- DOI: 10.1007/s12195-016-0456-5
Cholesterol Enrichment Impairs Capacitative Calcium Entry, eNOS Phosphorylation & Shear Stress-Induced NO Production
Abstract
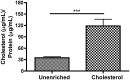
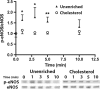
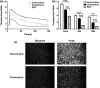
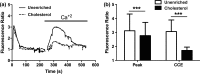
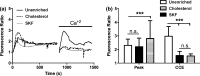
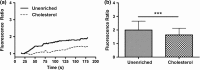
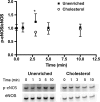
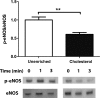
Endothelial dysfunction, characterized by decreased production or availability of nitric oxide (NO), is widely believed to be the hallmark of early-stage atherosclerosis. In addition, hypercholesterolemia is considered a major risk factor for development of atherosclerosis and is associated with impaired flow-induced dilation. However, the mechanism by which elevated cholesterol levels leads to decreased production of NO is unclear. NO is released in response to shear stress and agonist-evoked changes in intracellular calcium. Although calcium signaling is complex, we have previously shown that NO production by endothelial nitric oxide synthase (eNOS) is preferentially activated by calcium influx via store-operated channels. We hypothesized that cholesterol enrichment altered this signaling pathway (known as capacitive calcium entry; CCE) ultimately leading to decreased NO. Our results show that cholesterol enrichment abolished ATP-induced eNOS phosphorylation and attenuated the calcium response by the preferential inhibition of CCE. Furthermore, cholesterol enrichment also inhibited shear stress-induced NO production and eNOS phosporylation, consistent with our previous results showing a significant role for ATP autocrine stimulation and subsequent activation of CCE in the endothelial flow response.
Keywords: Atherosclerosis; Capacitative calcium entry; Cholesterol; Endothelial cells; Nitric oxide; Shear stress.
Conflict of interest statement
Ms. Muzorewa, Ms. Zaccheo, and Dr. Buerk have nothing to disclose. Dr. Andrews, Dr. Jaron and Dr. Barbee have a patent 8,828,711 issued.
Figures
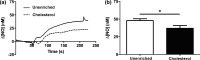
Similar articles
-
Shear stress-induced NO production is dependent on ATP autocrine signaling and capacitative calcium entry.Cell Mol Bioeng. 2014 Dec 1;7(4):510-520. doi: 10.1007/s12195-014-0351-x. Cell Mol Bioeng. 2014. PMID: 25386222 Free PMC article.
-
Effect of spatial heterogeneity and colocalization of eNOS and capacitative calcium entry channels on shear stress-induced NO production by endothelial cells: A modeling approach.Cell Mol Bioeng. 2018 Apr;11(2):143-155. doi: 10.1007/s12195-018-0520-4. Epub 2018 Mar 19. Cell Mol Bioeng. 2018. PMID: 30288177 Free PMC article.
-
Effects of pulsatile shear stress on signaling mechanisms controlling nitric oxide production, endothelial nitric oxide synthase phosphorylation, and expression in ovine fetoplacental artery endothelial cells.Endothelium. 2005 Jan-Apr;12(1-2):21-39. doi: 10.1080/10623320590933743. Endothelium. 2005. PMID: 16036314
-
Role of Ca2+ signaling in the regulation of endothelial permeability.Vascul Pharmacol. 2002 Nov;39(4-5):173-85. doi: 10.1016/s1537-1891(03)00007-7. Vascul Pharmacol. 2002. PMID: 12747958 Review.
-
Regulation of capacitative and non-capacitative Ca2+ entry in A7r5 vascular smooth muscle cells.Biol Res. 2004;37(4):641-5. doi: 10.4067/s0716-97602004000400020. Biol Res. 2004. PMID: 15709692 Review.
Cited by
-
Molecular chaperones in the brain endothelial barrier: neurotoxicity or neuroprotection?FASEB J. 2019 Nov;33(11):11629-11639. doi: 10.1096/fj.201900895R. Epub 2019 Jul 26. FASEB J. 2019. PMID: 31348679 Free PMC article. Review.
-
[Risk factors for recurrence of large atherosclerotic cerebral infarction].Nan Fang Yi Ke Da Xue Xue Bao. 2017 Dec 20;37(12):1678-1682. doi: 10.3969/j.issn.1673-4254.2017.12.20. Nan Fang Yi Ke Da Xue Xue Bao. 2017. PMID: 29292265 Free PMC article. Chinese.
-
Effects of Antirheumatic Treatment on Cell Cholesterol Efflux and Loading Capacity of Serum Lipoproteins in Spondylarthropathies.J Clin Med. 2022 Dec 9;11(24):7330. doi: 10.3390/jcm11247330. J Clin Med. 2022. PMID: 36555946 Free PMC article.
-
Immune cells and inflammatory mediators cause endothelial dysfunction in a vascular microphysiological system.Lab Chip. 2024 Mar 12;24(6):1808-1820. doi: 10.1039/d3lc00824j. Lab Chip. 2024. PMID: 38363157
-
TRPC channel-derived calcium fluxes differentially regulate ATP and flow-induced activation of eNOS.Nitric Oxide. 2021 Jun 1;111-112:1-13. doi: 10.1016/j.niox.2021.03.005. Epub 2021 Apr 1. Nitric Oxide. 2021. PMID: 33813098 Free PMC article.
References
-
- Bialecki RA, Tulenko TN. Excess membrane cholesterol alters calcium channels in arterial smooth muscle. Am. J. Physiol. 1989;257:C306–C314. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

