SCA-1 Labels a Subset of Estrogen-Responsive Bipotential Repopulating Cells within the CD24+ CD49fhi Mammary Stem Cell-Enriched Compartment
- PMID: 28132885
- PMCID: PMC5312257
- DOI: 10.1016/j.stemcr.2016.12.022
SCA-1 Labels a Subset of Estrogen-Responsive Bipotential Repopulating Cells within the CD24+ CD49fhi Mammary Stem Cell-Enriched Compartment
Abstract
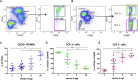
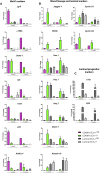
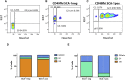
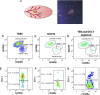
Estrogen stimulates breast development during puberty and mammary tumors in adulthood through estrogen receptor-α (ERα). These effects are proposed to occur via ERα+ luminal cells and not the mammary stem cells (MaSCs) that are ERαneg. Since ERα+ luminal cells express stem cell antigen-1 (SCA-1), we sought to determine if SCA-1 could define an ERα+ subset of EpCAM+/CD24+/CD49fhi MaSCs. We show that the MaSC population has a distinct SCA-1+ population that is abundant in pre-pubertal mammary glands. The SCA-1+ MaSCs have less stem cell markers and less in vivo repopulating activity than their SCA-1neg counterparts. However, they express ERα and specifically enter the cell cycle at puberty. Using estrogen-deficient aromatase knockouts (ArKO), we showed that the SCA-1+ MaSC could be directly modulated by estrogen supplementation. Thus, SCA-1 enriches for an ERα+, estrogen-sensitive subpopulation within the CD24+/CD49fhi MaSC population that may be responsible for the hormonal sensitivity of the developing mammary gland.
Keywords: Sca-1; estrogen; mammary stem cells.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
CD24 and CD49f expressions of E14.5 mouse mammary anlagen cells define putative distribution of earlier embryonic mammary stem cell activities.Biochem Cell Biol. 2018 Oct;96(5):539-547. doi: 10.1139/bcb-2017-0177. Epub 2018 Apr 5. Biochem Cell Biol. 2018. PMID: 29620414
-
Molecular signature of the putative stem/progenitor cells committed to the development of the bovine mammary gland at puberty.Sci Rep. 2018 Nov 1;8(1):16194. doi: 10.1038/s41598-018-34691-2. Sci Rep. 2018. PMID: 30385815 Free PMC article.
-
Separation by cell size enriches for mammary stem cell repopulation activity.Stem Cells Transl Med. 2013 Mar;2(3):199-203. doi: 10.5966/sctm.2012-0121. Epub 2013 Feb 13. Stem Cells Transl Med. 2013. PMID: 23408103 Free PMC article.
-
Mammary gland stem cells: more puzzles than explanations.J Biosci. 2012 Jun;37(2):349-58. doi: 10.1007/s12038-012-9200-z. J Biosci. 2012. PMID: 22581339 Review.
-
Mammary stem cells and the differentiation hierarchy: current status and perspectives.Genes Dev. 2014 Jun 1;28(11):1143-58. doi: 10.1101/gad.242511.114. Genes Dev. 2014. PMID: 24888586 Free PMC article. Review.
Cited by
-
Functional and Phenotypic Characterisations of Common Syngeneic Tumour Cell Lines as Estrogen Receptor-Positive Breast Cancer Models.Int J Mol Sci. 2023 Mar 16;24(6):5666. doi: 10.3390/ijms24065666. Int J Mol Sci. 2023. PMID: 36982737 Free PMC article.
-
Molecular Mechanisms of IL18 in Disease.Int J Mol Sci. 2023 Dec 6;24(24):17170. doi: 10.3390/ijms242417170. Int J Mol Sci. 2023. PMID: 38139000 Free PMC article. Review.
-
Emerging Role of Lymphocyte Antigen-6 Family of Genes in Cancer and Immune Cells.Front Immunol. 2019 Apr 24;10:819. doi: 10.3389/fimmu.2019.00819. eCollection 2019. Front Immunol. 2019. PMID: 31068932 Free PMC article. Review.
-
Estrogen Effects on the Mammary Gland in Early and Late Life and Breast Cancer Risk.Front Oncol. 2017 May 26;7:110. doi: 10.3389/fonc.2017.00110. eCollection 2017. Front Oncol. 2017. PMID: 28603694 Free PMC article. Review.
-
The benefits of adipocyte metabolism in bone health and regeneration.Front Cell Dev Biol. 2023 Feb 21;11:1104709. doi: 10.3389/fcell.2023.1104709. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36895792 Free PMC article. Review.
References
-
- Asselin-Labat M.-L., Shackleton M., Stingl J., Vaillant F., Forrest N.C., Eaves C.J., Visvader J.E., Lindeman G.J. Steroid hormone receptor status of mouse mammary stem cells. J. Natl. Cancer Inst. 2006;98:1011–1014. - PubMed
-
- Asselin-Labat M.L., Vaillant F., Sheridan J.M., Pal B., Wu D., Simpson E.R., Yasuda H., Smyth G.K., Martin T.J., Lindeman G.J., Visvader J.E. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465:798–802. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

