The PIDDosome activates p53 in response to supernumerary centrosomes
- PMID: 28130345
- PMCID: PMC5287111
- DOI: 10.1101/gad.289728.116
The PIDDosome activates p53 in response to supernumerary centrosomes
Abstract
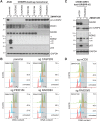
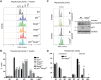
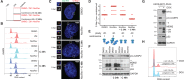
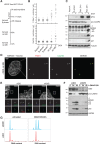
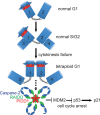
Centrosomes, the main microtubule-organizing centers in animal cells, are replicated exactly once during the cell division cycle to form the poles of the mitotic spindle. Supernumerary centrosomes can lead to aberrant cell division and have been causally linked to chromosomal instability and cancer. Here, we report that an increase in the number of mature centrosomes, generated by disrupting cytokinesis or forcing centrosome overduplication, triggers the activation of the PIDDosome multiprotein complex, leading to Caspase-2-mediated MDM2 cleavage, p53 stabilization, and p21-dependent cell cycle arrest. This pathway also restrains the extent of developmentally scheduled polyploidization by regulating p53 levels in hepatocytes during liver organogenesis. Taken together, the PIDDosome acts as a first barrier, engaging p53 to halt the proliferation of cells carrying more than one mature centrosome to maintain genome integrity.
Keywords: cell division; centrosome; cytokinesis failure; p53.
© 2017 Fava et al.; Published by Cold Spring Harbor Laboratory Press.
Figures


Comment in
-
Tumorigenesis: Extra! Extra! Read all about it!Nat Rev Cancer. 2017 Feb 23;17(3):143. doi: 10.1038/nrc.2017.12. Nat Rev Cancer. 2017. PMID: 28228642 No abstract available.
-
Centrosomes: PIDDosome Joins the Counting Game.Curr Biol. 2017 Mar 20;27(6):R237-R239. doi: 10.1016/j.cub.2017.02.001. Curr Biol. 2017. PMID: 28324744
Similar articles
-
Centriolar distal appendages activate the centrosome-PIDDosome-p53 signalling axis via ANKRD26.EMBO J. 2021 Feb 15;40(4):e104844. doi: 10.15252/embj.2020104844. Epub 2020 Dec 22. EMBO J. 2021. PMID: 33350486 Free PMC article.
-
ANKRD26 recruits PIDD1 to centriolar distal appendages to activate the PIDDosome following centrosome amplification.EMBO J. 2021 Feb 15;40(4):e105106. doi: 10.15252/embj.2020105106. Epub 2020 Dec 22. EMBO J. 2021. PMID: 33350495 Free PMC article.
-
The resurrection of the PIDDosome - emerging roles in the DNA-damage response and centrosome surveillance.J Cell Sci. 2017 Nov 15;130(22):3779-3787. doi: 10.1242/jcs.203448. J Cell Sci. 2017. PMID: 29142064 Review.
-
E2F-Family Members Engage the PIDDosome to Limit Hepatocyte Ploidy in Liver Development and Regeneration.Dev Cell. 2020 Feb 10;52(3):335-349.e7. doi: 10.1016/j.devcel.2019.12.016. Epub 2020 Jan 23. Dev Cell. 2020. PMID: 31983631
-
Total recall: the role of PIDDosome components in neurodegeneration.Trends Mol Med. 2023 Dec;29(12):996-1013. doi: 10.1016/j.molmed.2023.08.008. Epub 2023 Sep 14. Trends Mol Med. 2023. PMID: 37716905 Review.
Cited by
-
The PIDDosome: centrosome guardian and backup on the DNA damage response.Mol Cell Oncol. 2021 Mar 28;8(3):1893625. doi: 10.1080/23723556.2021.1893625. eCollection 2021. Mol Cell Oncol. 2021. PMID: 34027036 Free PMC article.
-
Validation of the Intermolecular Disulfide Bond in Caspase-2.Biology (Basel). 2024 Jan 17;13(1):49. doi: 10.3390/biology13010049. Biology (Basel). 2024. PMID: 38248479 Free PMC article.
-
Making the head: Caspases in life and death.Front Cell Dev Biol. 2023 Jan 13;10:1075751. doi: 10.3389/fcell.2022.1075751. eCollection 2022. Front Cell Dev Biol. 2023. PMID: 36712975 Free PMC article. Review.
-
The variant senescence-associated secretory phenotype induced by centrosome amplification constitutes a pathway that activates hypoxia-inducible factor-1α.Aging Cell. 2023 Mar;22(3):e13766. doi: 10.1111/acel.13766. Epub 2023 Jan 20. Aging Cell. 2023. PMID: 36660875 Free PMC article.
-
Septin and Ras regulate cytokinetic abscission in detached cells.Cell Div. 2019 Aug 21;14:8. doi: 10.1186/s13008-019-0051-y. eCollection 2019. Cell Div. 2019. PMID: 31452675 Free PMC article.
References
-
- Baliga BC, Read SH, Kumar S. 2004. The biochemical mechanism of caspase-2 activation. Cell Death Differ 11: 1234–1241. - PubMed
-
- Castedo M, Perfettini J-L, Roumier T, Valent A, Raslova H, Yakushijin K, Horne D, Feunteun J, Lenoir G, Medema R, et al. 2004. Mitotic catastrophe constitutes a special case of apoptosis whose suppression entails aneuploidy. Oncogene 23: 4362–4370. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
