Where no Ras has gone before: VPS35 steers N-Ras through the cytosol
- PMID: 28129035
- PMCID: PMC6343534
- DOI: 10.1080/21541248.2016.1263380
Where no Ras has gone before: VPS35 steers N-Ras through the cytosol
Abstract
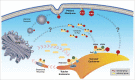
Ras is the best-studied member of the superfamily of small GTPases because of its role in cancer. Ras proteins transmit signals for proliferation, differentiation and survival. Three RAS genes encode 4 isoforms. All Ras isoforms have long been considered membrane bound, a localization required for function. Our recent study revealed that N-Ras differs from all other isoforms in being largely cytosolic even following modification with a prenyl lipid. Endogenous, cytosolic N-Ras chromatographed in both high and low molecular weight pools, a pattern that required prenylation, suggesting prenyl-dependent interaction with other proteins. VPS35, a coat protein of the retromer, was shown to interact with prenylated N-Ras in the cytosol. Silencing VPS35 results in partial N-Ras mislocalization on vesicular and tubulovesicular structures, reduced GTP-loading of Ras proteins, and inhibited proliferation and MAPK signaling in an oncogenic N-Ras-driven tumor cell line. Our data revealed a novel regulator of N-Ras trafficking and signaling.
Keywords: N-Ras; Ras; VPS35; cytosolic protein; oncogene; prenylation; trafficking.
Figures
Similar articles
-
VPS35 binds farnesylated N-Ras in the cytosol to regulate N-Ras trafficking.J Cell Biol. 2016 Aug 15;214(4):445-58. doi: 10.1083/jcb.201604061. Epub 2016 Aug 8. J Cell Biol. 2016. PMID: 27502489 Free PMC article.
-
A novel statin-mediated "prenylation block-and-release" assay provides insight into the membrane targeting mechanisms of small GTPases.Biochem Biophys Res Commun. 2010 Jun 18;397(1):34-41. doi: 10.1016/j.bbrc.2010.05.045. Epub 2010 May 13. Biochem Biophys Res Commun. 2010. PMID: 20471365 Free PMC article.
-
Prenylation is not necessary for endogenous Ras activation in non-malignant cells.J Cell Biochem. 2006 Feb 1;97(2):412-22. doi: 10.1002/jcb.20641. J Cell Biochem. 2006. PMID: 16187291
-
Membrane association and targeting of prenylated Ras-like GTPases.Cell Signal. 1998 Mar;10(3):167-72. doi: 10.1016/s0898-6568(97)00120-4. Cell Signal. 1998. PMID: 9607139 Review.
-
The role of palmitoylation in regulating Ras localization and function.Biochem Soc Trans. 2013 Feb 1;41(1):79-83. doi: 10.1042/BST20120268. Biochem Soc Trans. 2013. PMID: 23356262 Review.
Cited by
-
DNA and RNA sequencing identified a novel oncogene VPS35 in liver hepatocellular carcinoma.Oncogene. 2020 Apr;39(16):3229-3244. doi: 10.1038/s41388-020-1215-6. Epub 2020 Feb 19. Oncogene. 2020. PMID: 32071398
-
Rsr1 Palmitoylation and GTPase Activity Status Differentially Coordinate Nuclear, Septin, and Vacuole Dynamics in Candida albicans.mBio. 2020 Oct 13;11(5):e01666-20. doi: 10.1128/mBio.01666-20. mBio. 2020. PMID: 33051364 Free PMC article.
-
Diffuse midline glioma with novel, potentially targetable, FGFR2-VPS35 fusion.Cold Spring Harb Mol Case Stud. 2020 Oct 7;6(5):a005660. doi: 10.1101/mcs.a005660. Print 2020 Oct. Cold Spring Harb Mol Case Stud. 2020. PMID: 32839179 Free PMC article.
-
Genetic disruption of N-RasG12D palmitoylation perturbs hematopoiesis and prevents myeloid transformation in mice.Blood. 2020 May 14;135(20):1772-1782. doi: 10.1182/blood.2019003530. Blood. 2020. PMID: 32219446 Free PMC article.
References
-
- Wright LP, Philips MR. Thematic review series: lipid posttranslational modifications. CAAX modification and membrane targeting of Ras. J Lipid Res 2006; 47:883-91; PMID:16543601; http://dx.doi.org/10.1194/jlr.R600004-JLR200 - DOI - PubMed
-
- Hancock JF, Magee AI, Childs JE, Marshall CJ. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell 1989; 57:1167-77; PMID:2661017; http://dx.doi.org/10.1016/0092-8674(89)90054-8 - DOI - PubMed
-
- Schafer WR, Trueblood CE, Yang C, Mayer MP, Rosenberg S, Poulter CD, Kim SH, Rine J. Enzymatic coupling of cholesterol intermediates to a mating pheromone precursor and to the ras protein. Science 1990; 249:1133-9; PMID:2204115; http://dx.doi.org/10.1126/science.2204115 - DOI - PubMed
-
- Boyartchuk VL, Ashby MN, Rine J. Modulation of Ras and a-Factor Function by Carboxyl-Terminal Proteolysis. Science 1997; 275:1796-800; PMID:9065405; http://dx.doi.org/10.1126/science.275.5307.1796 - DOI - PubMed
-
- Otto JC, Kim E, Young SG, Casey PJ. Cloning and characterization of a mammalian prenyl protein-specific protease. J Biol Chem 1999; 274:8379-82; PMID:10085068; http://dx.doi.org/10.1074/jbc.274.13.8379 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
