Metabolic zonation of the liver: The oxygen gradient revisited
- PMID: 28126520
- PMCID: PMC5257182
- DOI: 10.1016/j.redox.2017.01.012
Metabolic zonation of the liver: The oxygen gradient revisited
Abstract
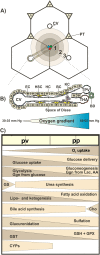
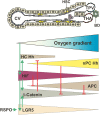
The liver has a multitude of functions which are necessary to maintain whole body homeostasis. This requires that various metabolic pathways can run in parallel in the most efficient manner and that futile cycles are kept to a minimum. To a large extent this is achieved due to a functional specialization of the liver parenchyma known as metabolic zonation which is often lost in liver diseases. Although this phenomenon is known for about 40 years, the underlying regulatory pathways are not yet fully elucidated. The physiologically occurring oxygen gradient was considered to be crucial for the appearance of zonation; however, a number of reports during the last decade indicating that β-catenin signaling, and the hedgehog (Hh) pathway contribute to metabolic zonation may have shifted this view. In the current review we connect these new observations with the concept that the oxygen gradient within the liver acinus is a regulator of zonation. This is underlined by a number of facts showing that the β-catenin and the Hh pathway can be modulated by the hypoxia signaling system and the hypoxia-inducible transcription factors (HIFs). Altogether, we provide a view by which the dynamic interplay between all these pathways can drive liver zonation and thus contribute to its physiological function.
Keywords: Hepatocytes; Hypoxia, HIF, Liver; Metabolic zonation; Metabolism; Morphogen signaling; Optimization; Pathology; ROS, Antioxidants, Antioxidative enzymes, Matrix, Diet, Fibrosis, Homeostasis; Regulatory network; Sinusoid.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Liver Zonation in Health and Disease: Hypoxia and Hypoxia-Inducible Transcription Factors as Concert Masters.Int J Mol Sci. 2019 May 11;20(9):2347. doi: 10.3390/ijms20092347. Int J Mol Sci. 2019. PMID: 31083568 Free PMC article. Review.
-
β-catenin links hepatic metabolic zonation with lipid metabolism and diet-induced obesity in mice.Am J Pathol. 2014 Dec;184(12):3284-98. doi: 10.1016/j.ajpath.2014.08.022. Epub 2014 Oct 7. Am J Pathol. 2014. PMID: 25300578 Free PMC article.
-
Pre-clinical and clinical investigations of metabolic zonation in liver diseases: The potential of microphysiology systems.Exp Biol Med (Maywood). 2017 Oct;242(16):1605-1616. doi: 10.1177/1535370217707731. Epub 2017 May 3. Exp Biol Med (Maywood). 2017. PMID: 28467181 Free PMC article. Review.
-
The Canonical Wnt Pathway as a Key Regulator in Liver Development, Differentiation and Homeostatic Renewal.Genes (Basel). 2020 Sep 30;11(10):1163. doi: 10.3390/genes11101163. Genes (Basel). 2020. PMID: 33008122 Free PMC article. Review.
-
Transcription dynamics in a physiological process: β-catenin signaling directs liver metabolic zonation.Int J Biochem Cell Biol. 2011 Feb;43(2):271-8. doi: 10.1016/j.biocel.2009.11.004. Epub 2009 Nov 13. Int J Biochem Cell Biol. 2011. PMID: 19914393 Review.
Cited by
-
Multiple Facets of Cellular Homeostasis and Regeneration of the Mammalian Liver.Annu Rev Physiol. 2023 Feb 10;85:469-493. doi: 10.1146/annurev-physiol-032822-094134. Epub 2022 Oct 21. Annu Rev Physiol. 2023. PMID: 36270290 Free PMC article. Review.
-
Mitochondrial topoisomerase I (Top1MT) prevents the onset of metabolic dysfunction-associated steatohepatitis (MASH) in mice.bioRxiv [Preprint]. 2024 Sep 5:2024.09.05.611454. doi: 10.1101/2024.09.05.611454. bioRxiv. 2024. PMID: 39372760 Free PMC article. Preprint.
-
Lobular distribution of enhanced expression levels of heat shock proteins using in-situ hybridization in the mouse liver treated with a single administration of CCl4.J Toxicol Pathol. 2024 Jan;37(1):29-37. doi: 10.1293/tox.2023-0053. Epub 2023 Oct 27. J Toxicol Pathol. 2024. PMID: 38283376 Free PMC article.
-
Physiologically relevant oxygen tensions differentially regulate hepatotoxic responses in HepG2 cells.Toxicol In Vitro. 2021 Aug;74:105156. doi: 10.1016/j.tiv.2021.105156. Epub 2021 Mar 31. Toxicol In Vitro. 2021. PMID: 33811995 Free PMC article.
-
Oxygen Gradient Induced in Microfluidic Chips Can Be Used as a Model for Liver Zonation.Cells. 2022 Nov 23;11(23):3734. doi: 10.3390/cells11233734. Cells. 2022. PMID: 36496994 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

