The golgin coiled-coil proteins capture different types of transport carriers via distinct N-terminal motifs
- PMID: 28122620
- PMCID: PMC5267433
- DOI: 10.1186/s12915-016-0345-3
The golgin coiled-coil proteins capture different types of transport carriers via distinct N-terminal motifs
Abstract
Background: The internal organization of cells depends on mechanisms to ensure that transport carriers, such as vesicles, fuse only with the correct destination organelle. Several types of proteins have been proposed to confer specificity to this process, and we have recently shown that a set of coiled-coil proteins on the Golgi, called golgins, are able to capture specific classes of carriers when relocated to an ectopic location.
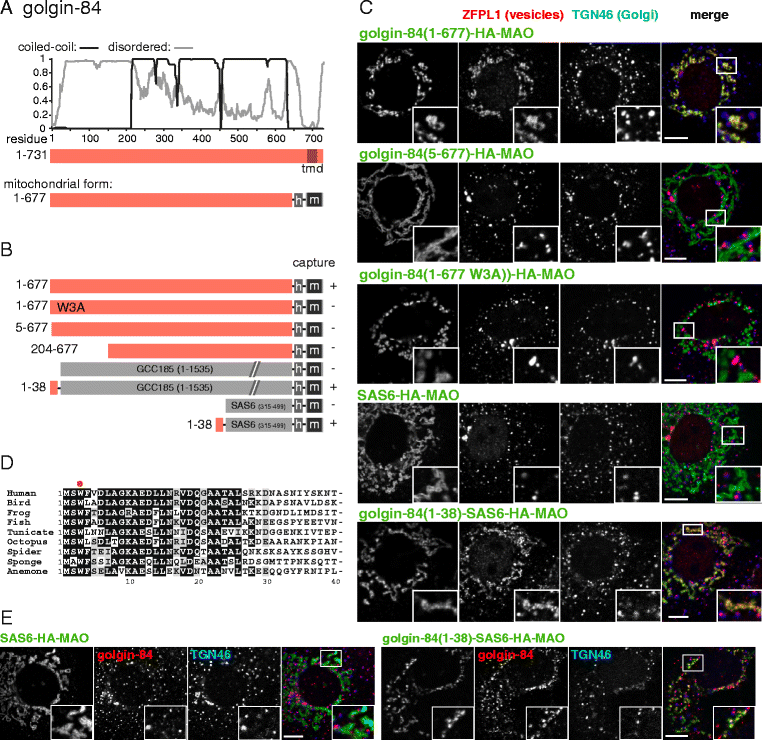
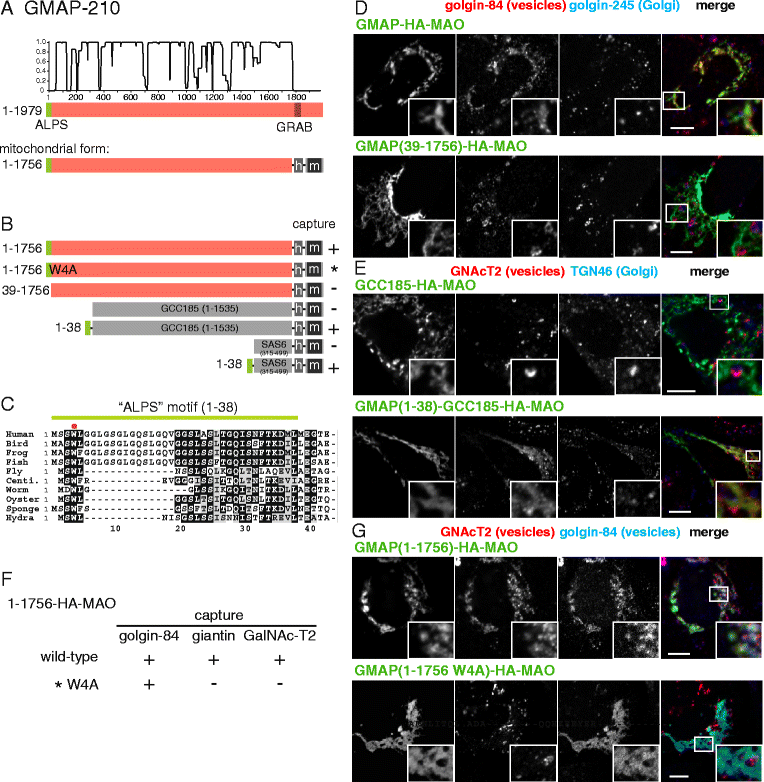
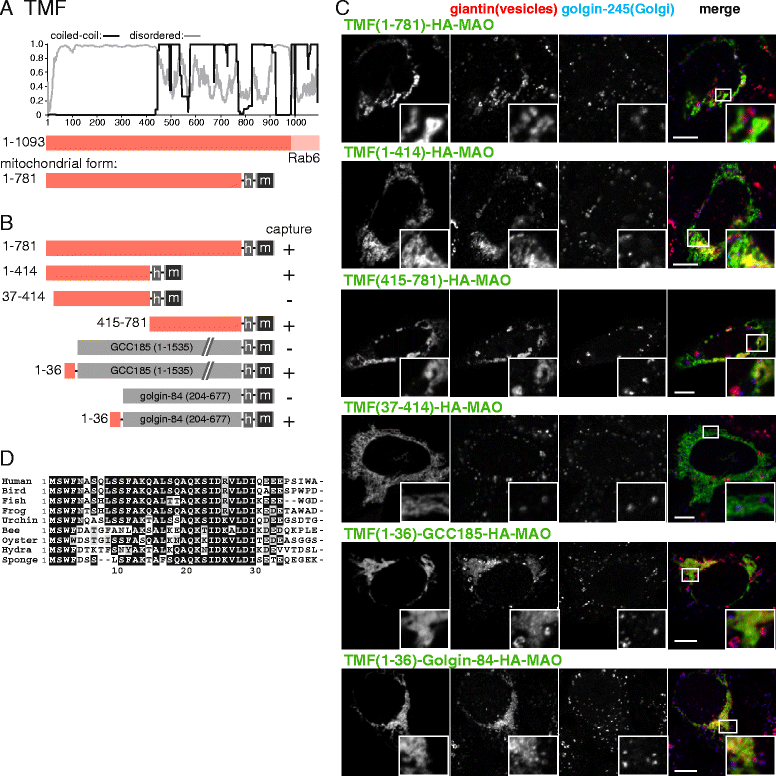
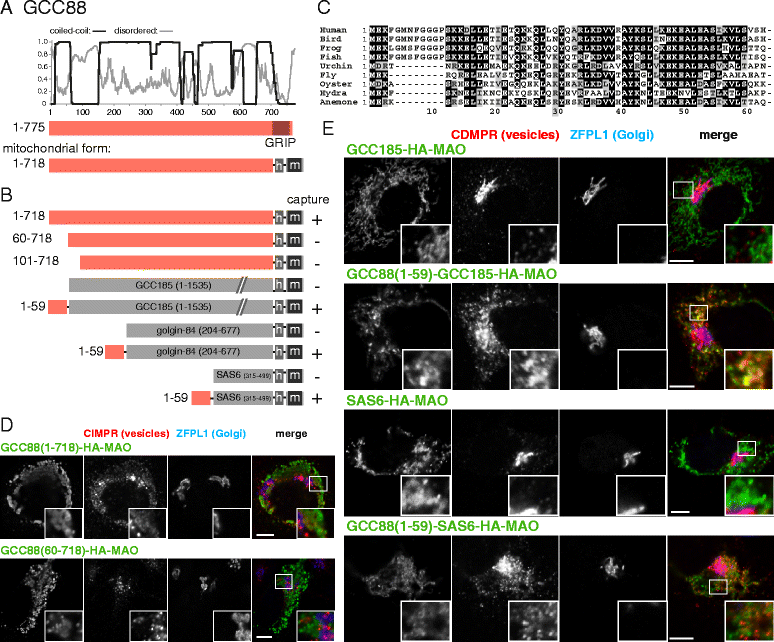
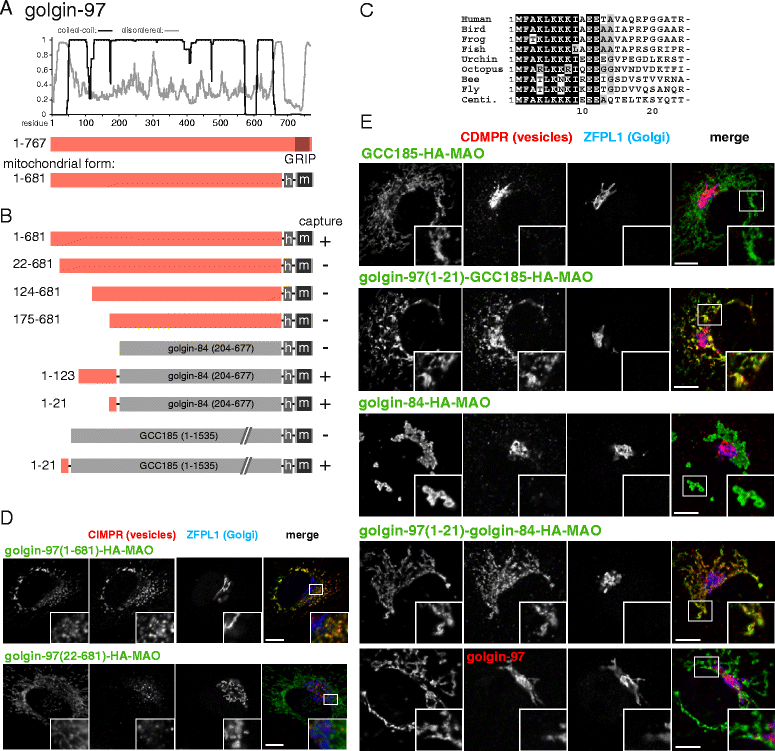
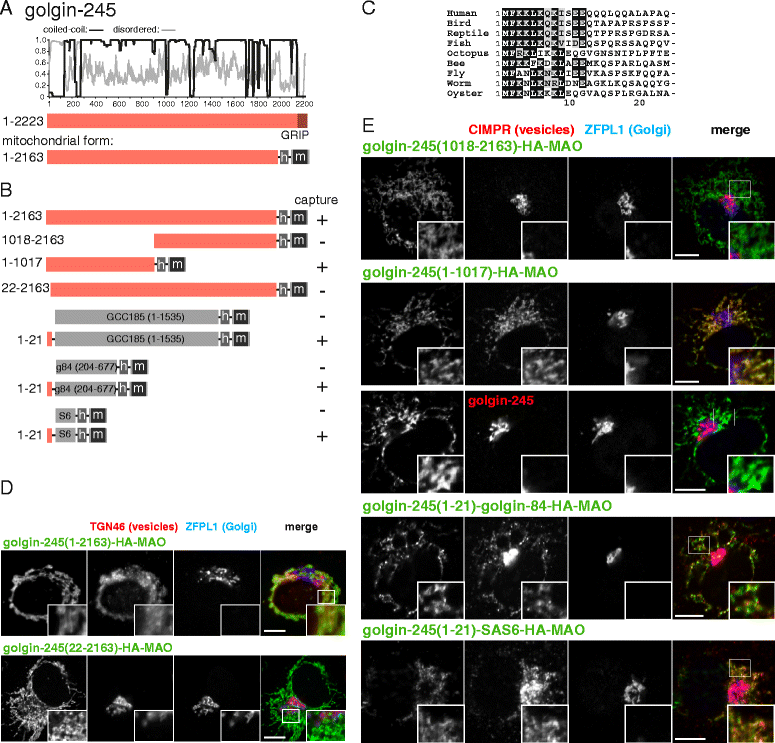
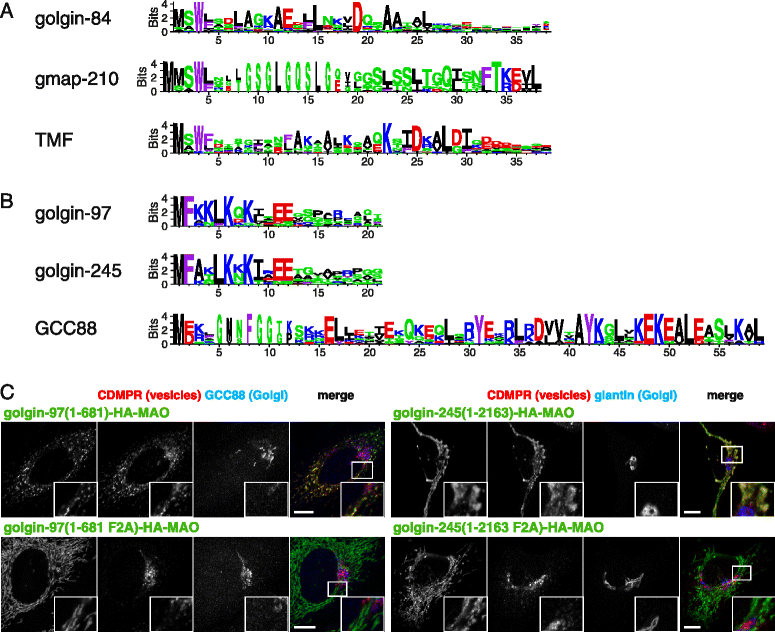
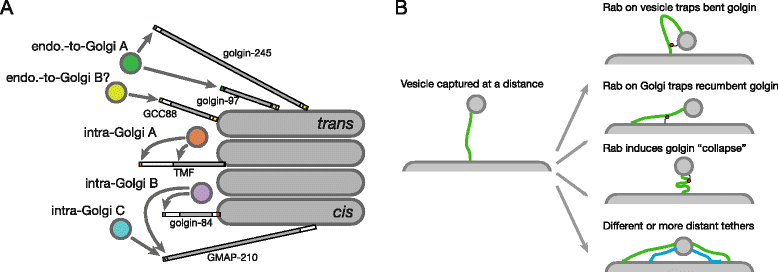
Results: Mapping of six different golgins reveals that, in each case, a short 20-50 residue region is necessary and sufficient to capture specific carriers. In all six of GMAP-210, golgin-84, TMF, golgin-97, golgin-245, and GCC88, this region is located at the extreme N-terminus of the protein. The vesicle-capturing regions of GMAP-210, golgin-84, and TMF capture intra-Golgi vesicles and share some sequence features, suggesting that they act in a related, if distinct, manner. In the case of GMAP-210, this shared feature is in addition to a previously characterized "amphipathic lipid-packing sensor" motif that can capture highly curved membranes, with the two motifs being apparently involved in capturing distinct types of vesicles. Of the three GRIP domain golgins that capture endosome-to-Golgi carriers, golgin-97 and golgin-245 share a closely related capture motif, whereas that in GCC88 is distinct, suggesting that it works by a different mechanism and raising the possibility that the three golgins capture different classes of endosome-derived carriers that share many cargos but have distinct features for recognition at the Golgi.
Conclusions: For six different golgins, the capture of carriers is mediated by a short region at the N-terminus of the protein. There appear to be at least four different types of motif, consistent with specific golgins capturing specific classes of carrier and implying the existence of distinct receptors present on each of these different carrier classes.
Keywords: Coiled-coil; Endosome-to-Golgi traffic; Golgi; Golgin; Intra-Golgi traffic; Vesicle tethering.
Figures
Similar articles
-
In vivo characterization of Drosophila golgins reveals redundancy and plasticity of vesicle capture at the Golgi apparatus.Curr Biol. 2022 Nov 7;32(21):4549-4564.e6. doi: 10.1016/j.cub.2022.08.054. Epub 2022 Sep 13. Curr Biol. 2022. PMID: 36103876 Free PMC article.
-
TBC1D23 is a bridging factor for endosomal vesicle capture by golgins at the trans-Golgi.Nat Cell Biol. 2017 Dec;19(12):1424-1432. doi: 10.1038/ncb3627. Epub 2017 Oct 30. Nat Cell Biol. 2017. PMID: 29084197 Free PMC article.
-
A novel Rab6-interacting domain defines a family of Golgi-targeted coiled-coil proteins.Curr Biol. 1999 Apr 8;9(7):381-4. doi: 10.1016/s0960-9822(99)80167-5. Curr Biol. 1999. PMID: 10209123
-
Recognition and tethering of transport vesicles at the Golgi apparatus.Curr Opin Cell Biol. 2017 Aug;47:16-23. doi: 10.1016/j.ceb.2017.02.003. Epub 2017 Feb 24. Curr Opin Cell Biol. 2017. PMID: 28237810 Review.
-
Finding the Golgi: Golgin Coiled-Coil Proteins Show the Way.Trends Cell Biol. 2016 Jun;26(6):399-408. doi: 10.1016/j.tcb.2016.02.005. Epub 2016 Mar 11. Trends Cell Biol. 2016. PMID: 26972448 Review.
Cited by
-
In vivo characterization of Drosophila golgins reveals redundancy and plasticity of vesicle capture at the Golgi apparatus.Curr Biol. 2022 Nov 7;32(21):4549-4564.e6. doi: 10.1016/j.cub.2022.08.054. Epub 2022 Sep 13. Curr Biol. 2022. PMID: 36103876 Free PMC article.
-
Structural Organization and Function of the Golgi Ribbon During Cell Division.Front Cell Dev Biol. 2022 Jun 24;10:925228. doi: 10.3389/fcell.2022.925228. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35813197 Free PMC article.
-
Spatial proteomics defines the content of trafficking vesicles captured by golgin tethers.Nat Commun. 2020 Nov 25;11(1):5987. doi: 10.1038/s41467-020-19840-4. Nat Commun. 2020. PMID: 33239640 Free PMC article.
-
The golgin family exhibits a propensity to form condensates in living cells.FEBS Lett. 2020 Oct;594(19):3086-3094. doi: 10.1002/1873-3468.13884. Epub 2020 Aug 2. FEBS Lett. 2020. PMID: 32668013 Free PMC article.
-
Golgin-97 Targets Ectopically Expressed Inward Rectifying Potassium Channel, Kir2.1, to the trans-Golgi Network in COS-7 Cells.Front Physiol. 2018 Aug 3;9:1070. doi: 10.3389/fphys.2018.01070. eCollection 2018. Front Physiol. 2018. PMID: 30123141 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

