NLRP3 inflammasome: Pathogenic role and potential therapeutic target for IgA nephropathy
- PMID: 28117341
- PMCID: PMC5259731
- DOI: 10.1038/srep41123
NLRP3 inflammasome: Pathogenic role and potential therapeutic target for IgA nephropathy
Abstract
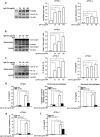
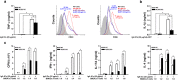
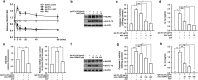
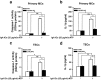
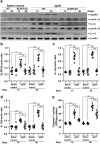
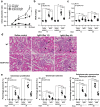
We have previously showed that IL-1β is involved in the pathogenesis of both spontaneously occurring and passively induced IgA nephropathy (IgAN) models. However, the exact causal-relationship between NLRP3 inflammasome and the pathogenesis of IgAN remains unknown. In the present study, we showed that [1] IgA immune complexes (ICs) activated NLRP3 inflammasome in macrophages involving disruption of mitochondrial integrity and induction of mitochondrial ROS, bone marrow-derived dendritic cells (BMDCs) and renal intrinsic cells; [2] knockout of NLRP3 inhibited IgA ICs-mediated activation of BMDCs and T cells; and [3] knockout of NLRP3 or a kidney-targeting delivery of shRNA of NLRP3 improved renal function and renal injury in a mouse IgAN model. These results strongly suggest that NLRP3 inflammasome serves as a key player in the pathogenesis of IgAN partly through activation of T cells and mitochondrial ROS production and that a local, kidney-targeting suppression of NLRP3 be a therapeutic strategy for IgAN.
Figures

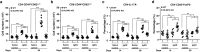



Similar articles
-
The Role of NLRP3 Inflammasome in IgA Nephropathy.Medicina (Kaunas). 2022 Dec 30;59(1):82. doi: 10.3390/medicina59010082. Medicina (Kaunas). 2022. PMID: 36676706 Free PMC article. Review.
-
Tris DBA ameliorates IgA nephropathy by blunting the activating signal of NLRP3 inflammasome through SIRT1- and SIRT3-mediated autophagy induction.J Cell Mol Med. 2020 Dec;24(23):13609-13622. doi: 10.1111/jcmm.15663. Epub 2020 Nov 1. J Cell Mol Med. 2020. PMID: 33135320 Free PMC article.
-
Antroquinonol mitigates an accelerated and progressive IgA nephropathy model in mice by activating the Nrf2 pathway and inhibiting T cells and NLRP3 inflammasome.Free Radic Biol Med. 2013 Aug;61:285-97. doi: 10.1016/j.freeradbiomed.2013.03.024. Epub 2013 Apr 6. Free Radic Biol Med. 2013. PMID: 23567192
-
Mitochondrial reactive oxygen species-mediated NLRP3 inflammasome activation contributes to aldosterone-induced renal tubular cells injury.Oncotarget. 2016 Apr 5;7(14):17479-91. doi: 10.18632/oncotarget.8243. Oncotarget. 2016. PMID: 27014913 Free PMC article.
-
The Role of Inflammasome-Dependent and Inflammasome-Independent NLRP3 in the Kidney.Cells. 2019 Nov 5;8(11):1389. doi: 10.3390/cells8111389. Cells. 2019. PMID: 31694192 Free PMC article. Review.
Cited by
-
The Role of NLRP3 Inflammasome in IgA Nephropathy.Medicina (Kaunas). 2022 Dec 30;59(1):82. doi: 10.3390/medicina59010082. Medicina (Kaunas). 2022. PMID: 36676706 Free PMC article. Review.
-
Involvement of Inflammasome Components in Kidney Disease.Antioxidants (Basel). 2022 Jan 27;11(2):246. doi: 10.3390/antiox11020246. Antioxidants (Basel). 2022. PMID: 35204131 Free PMC article. Review.
-
Renal macrophages and NLRP3 inflammasomes in kidney diseases and therapeutics.Cell Death Discov. 2024 May 13;10(1):229. doi: 10.1038/s41420-024-01996-3. Cell Death Discov. 2024. PMID: 38740765 Free PMC article. Review.
-
Novel insights into NOD-like receptors in renal diseases.Acta Pharmacol Sin. 2022 Nov;43(11):2789-2806. doi: 10.1038/s41401-022-00886-7. Epub 2022 Apr 1. Acta Pharmacol Sin. 2022. PMID: 35365780 Free PMC article. Review.
-
Interleukin-18 binding protein attenuates renal injury of adriamycin-induced mouse nephropathy.Int J Clin Exp Pathol. 2019 Aug 1;12(8):3005-3012. eCollection 2019. Int J Clin Exp Pathol. 2019. PMID: 31934138 Free PMC article.
References
-
- Rifai A. & Dworkin L. D. IgA nephropathy: markers of progression and clues to pathogenesis. Kidney Int. 73, 1338–1340 (2008). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

