Induction of dormancy in hypoxic human papillomavirus-positive cancer cells
- PMID: 28115701
- PMCID: PMC5307428
- DOI: 10.1073/pnas.1615758114
Induction of dormancy in hypoxic human papillomavirus-positive cancer cells
Abstract
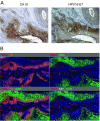
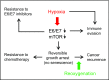
Oncogenic human papillomaviruses (HPVs) are closely linked to major human malignancies, including cervical and head and neck cancers. It is widely assumed that HPV-positive cancer cells are under selection pressure to continuously express the viral E6/E7 oncogenes, that their intracellular p53 levels are reconstituted on E6/E7 repression, and that E6/E7 inhibition phenotypically results in cellular senescence. Here we show that hypoxic conditions, as are often found in subregions of cervical and head and neck cancers, enable HPV-positive cancer cells to escape from these regulatory principles: E6/E7 is efficiently repressed, yet, p53 levels do not increase. Moreover, E6/E7 repression under hypoxia does not result in cellular senescence, owing to hypoxia-associated impaired mechanistic target of rapamycin (mTOR) signaling via the inhibitory REDD1/TSC2 axis. Instead, a reversible growth arrest is induced that can be overcome by reoxygenation. Impairment of mTOR signaling also interfered with the senescence response of hypoxic HPV-positive cancer cells toward prosenescent chemotherapy in vitro. Collectively, these findings indicate that hypoxic HPV-positive cancer cells can induce a reversible state of dormancy, with decreased viral antigen synthesis and increased therapeutic resistance, and may serve as reservoirs for tumor recurrence on reoxygenation.
Keywords: cervical cancer; human papillomavirus; hypoxia; mTOR; tumor virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
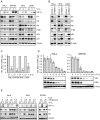
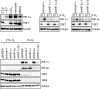
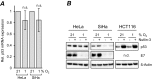
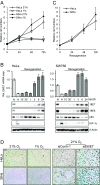

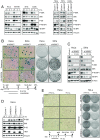
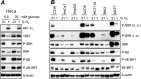
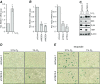

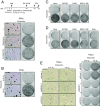

Comment in
-
Commentary: Induction of Dormancy in Hypoxic Human Papillomavirus-Positive Cancer Cells.Front Oncol. 2018 Mar 27;8:77. doi: 10.3389/fonc.2018.00077. eCollection 2018. Front Oncol. 2018. PMID: 29637045 Free PMC article. No abstract available.
Similar articles
-
Repression of Human Papillomavirus Oncogene Expression under Hypoxia Is Mediated by PI3K/mTORC2/AKT Signaling.mBio. 2019 Feb 12;10(1):e02323-18. doi: 10.1128/mBio.02323-18. mBio. 2019. PMID: 30755508 Free PMC article.
-
The HPV E6/E7 Oncogenes: Key Factors for Viral Carcinogenesis and Therapeutic Targets.Trends Microbiol. 2018 Feb;26(2):158-168. doi: 10.1016/j.tim.2017.07.007. Epub 2017 Aug 17. Trends Microbiol. 2018. PMID: 28823569 Review.
-
Repression of human papillomavirus oncogenes in HeLa cervical carcinoma cells causes the orderly reactivation of dormant tumor suppressor pathways.Proc Natl Acad Sci U S A. 2000 Nov 7;97(23):12513-8. doi: 10.1073/pnas.97.23.12513. Proc Natl Acad Sci U S A. 2000. PMID: 11070078 Free PMC article.
-
Effects of the antifungal agent ciclopirox in HPV-positive cancer cells: Repression of viral E6/E7 oncogene expression and induction of senescence and apoptosis.Int J Cancer. 2020 Jan 15;146(2):461-474. doi: 10.1002/ijc.32709. Epub 2019 Oct 31. Int J Cancer. 2020. PMID: 31603527
-
Virus/Host Cell Crosstalk in Hypoxic HPV-Positive Cancer Cells.Viruses. 2017 Jul 5;9(7):174. doi: 10.3390/v9070174. Viruses. 2017. PMID: 28678198 Free PMC article. Review.
Cited by
-
Effects of hypoxia on antigen presentation and T cell-based immune recognition of HPV16-transformed cells.Front Immunol. 2022 Oct 21;13:918528. doi: 10.3389/fimmu.2022.918528. eCollection 2022. Front Immunol. 2022. PMID: 36341354 Free PMC article.
-
Human papillomavirus and genome instability: from productive infection to cancer.Clinics (Sao Paulo). 2018 Sep 6;73(suppl 1):e539s. doi: 10.6061/clinics/2018/e539s. Clinics (Sao Paulo). 2018. PMID: 30208168 Free PMC article. Review.
-
Modeling treatment-dependent glioma growth including a dormant tumor cell subpopulation.BMC Cancer. 2018 Apr 3;18(1):376. doi: 10.1186/s12885-018-4281-1. BMC Cancer. 2018. PMID: 29614985 Free PMC article.
-
FAM57A (Family with Sequence Similarity 57 Member A) Is a Cell-Density-Regulated Protein and Promotes the Proliferation and Migration of Cervical Cancer Cells.Cells. 2022 Oct 21;11(20):3309. doi: 10.3390/cells11203309. Cells. 2022. PMID: 36291175 Free PMC article.
-
Tracking HPV Infection, Associated Cancer Development, and Recent Treatment Efforts-A Comprehensive Review.Vaccines (Basel). 2023 Jan 1;11(1):102. doi: 10.3390/vaccines11010102. Vaccines (Basel). 2023. PMID: 36679945 Free PMC article. Review.
References
-
- zur Hausen H. Papillomaviruses and cancer: From basic studies to clinical application. Nat Rev Cancer. 2002;2(5):342–350. - PubMed
-
- Howley PM, Schiller JT, Lowy DR. Papillomaviruses. In: Knipe DM, Howley PM, editors. Fields Virology. 6th Ed. Lippincott, Williams & Wilkins; Philadelphia: 2013. pp. 1662–1703.
-
- American Cancer Society . Cancer Facts and Figures 2015. American Cancer Society; Atlanta, GA: 2015.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

