Unfolding anti-tumor immunity: ER stress responses sculpt tolerogenic myeloid cells in cancer
- PMID: 28105371
- PMCID: PMC5240216
- DOI: 10.1186/s40425-016-0203-4
Unfolding anti-tumor immunity: ER stress responses sculpt tolerogenic myeloid cells in cancer
Abstract
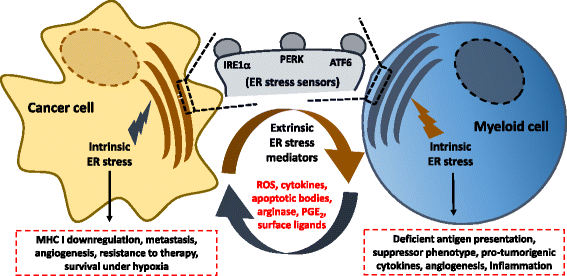
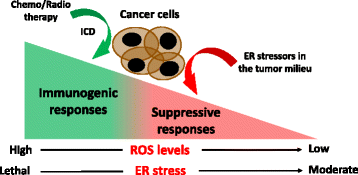
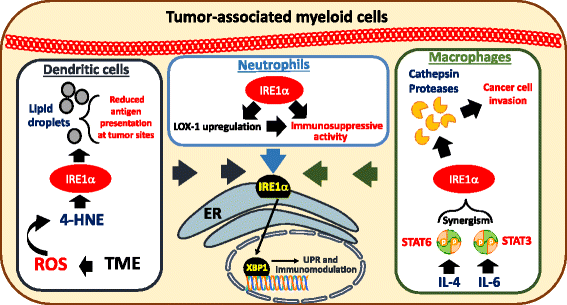
Established tumors build a stressful and hostile microenvironment that blocks the development of protective innate and adaptive immune responses. Different subsets of immunoregulatory myeloid populations, including dendritic cells, myeloid-derived suppressor cells (MDSCs) and macrophages, accumulate in the stressed tumor milieu and represent a major impediment to the success of various forms of cancer immunotherapy. Specific conditions and factors within tumor masses, including hypoxia, nutrient starvation, low pH, and increased levels of free radicals, provoke a state of "endoplasmic reticulum (ER) stress" in both malignant cells and infiltrating myeloid cells. In order to cope with ER stress, cancer cells and tumor-associated myeloid cells activate an integrated signaling pathway known as the Unfolded Protein Response (UPR), which promotes cell survival and adaptation under adverse environmental conditions. However, the UPR can also induce cell death under unresolved levels of ER stress. Three branches of the UPR have been described, including the activation of the inositol-requiring enzyme 1 (IRE1), the pancreatic ER kinase (PKR)-like ER kinase (PERK), and the activating transcription factor 6 (ATF6). In this minireview, we briefly discuss the role of ER stress and specific UPR mediators in tumor development, growth and metastasis. In addition, we describe how sustained ER stress responses operate as key mediators of chronic inflammation and immune suppression within tumors. Finally, we discuss multiple pharmacological approaches that overcome the immunosuppressive effect of the UPR in tumors, and that could potentially enhance the efficacy of cancer immunotherapies by reprogramming the function of tumor-infiltrating myeloid cells.
Keywords: CHOP; ER stress; IRE1; Immunotherapy; Myeloid cells; PERK; Tumor immunology; Unfolded Protein Responses; XBP1.
Figures
Similar articles
-
Sensing endoplasmic reticulum stress.Adv Exp Med Biol. 2012;738:153-68. doi: 10.1007/978-1-4614-1680-7_10. Adv Exp Med Biol. 2012. PMID: 22399379 Review.
-
Endoplasmic reticulum stress regulates tumor growth and anti-tumor immunity: a promising opportunity for cancer immunotherapy.Cancer Immunol Immunother. 2017 Aug;66(8):1069-1078. doi: 10.1007/s00262-017-2019-6. Epub 2017 Jun 2. Cancer Immunol Immunother. 2017. PMID: 28577085 Free PMC article. Review.
-
Mechanism of the induction of endoplasmic reticulum stress by the anti-cancer agent, di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT): Activation of PERK/eIF2α, IRE1α, ATF6 and calmodulin kinase.Biochem Pharmacol. 2016 Jun 1;109:27-47. doi: 10.1016/j.bcp.2016.04.001. Epub 2016 Apr 6. Biochem Pharmacol. 2016. PMID: 27059255
-
Targeting UPR branches, a potential strategy for enhancing efficacy of cancer chemotherapy.Acta Biochim Biophys Sin (Shanghai). 2021 Nov 10;53(11):1417-1427. doi: 10.1093/abbs/gmab131. Acta Biochim Biophys Sin (Shanghai). 2021. PMID: 34664059 Review.
-
PERK regulated miR-424(322)-503 cluster fine-tunes activation of IRE1 and ATF6 during Unfolded Protein Response.Sci Rep. 2015 Dec 17;5:18304. doi: 10.1038/srep18304. Sci Rep. 2015. PMID: 26674075 Free PMC article.
Cited by
-
Novel Combinatorial Approaches to Tackle the Immunosuppressive Microenvironment of Prostate Cancer.Cancers (Basel). 2021 Mar 8;13(5):1145. doi: 10.3390/cancers13051145. Cancers (Basel). 2021. PMID: 33800156 Free PMC article. Review.
-
Unfolded Protein Response Is Activated by Aurora Kinase A in Esophageal Adenocarcinoma.Cancers (Basel). 2022 Mar 9;14(6):1401. doi: 10.3390/cancers14061401. Cancers (Basel). 2022. PMID: 35326553 Free PMC article.
-
The Pathology of Morphine-Inhibited Nerve Repair and Morphine-Induced Nerve Damage Is Mediated via Endoplasmic Reticulum Stress.Front Neurosci. 2021 Feb 19;15:618190. doi: 10.3389/fnins.2021.618190. eCollection 2021. Front Neurosci. 2021. PMID: 33679302 Free PMC article.
-
Proteomic signatures of myeloid derived suppressor cells from liver and lung metastases reveal functional divergence and potential therapeutic targets.Cell Death Discov. 2021 Sep 4;7(1):232. doi: 10.1038/s41420-021-00621-x. Cell Death Discov. 2021. PMID: 34482371 Free PMC article.
-
Local Activation of p53 in the Tumor Microenvironment Overcomes Immune Suppression and Enhances Antitumor Immunity.Cancer Res. 2017 May 1;77(9):2292-2305. doi: 10.1158/0008-5472.CAN-16-2832. Epub 2017 Mar 9. Cancer Res. 2017. PMID: 28280037 Free PMC article.
References
-
- Chevet E, Hetz C, Samali A. Endoplasmic reticulum stress-activated cell reprogramming in oncogenesis. Cancer Discov. 2015;5:586–97. doi: 10.1158/2159-8290.CD-14-1490. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
