Hydrazone Linker as a Useful Tool for Preparing Chimeric Peptide/Nonpeptide Bifunctional Compounds
- PMID: 28105278
- PMCID: PMC5238479
- DOI: 10.1021/acsmedchemlett.6b00381
Hydrazone Linker as a Useful Tool for Preparing Chimeric Peptide/Nonpeptide Bifunctional Compounds
Abstract
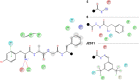
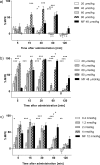
The area of multitarget compounds, joining two pharmacophores within one molecule, is a vivid field of research in medicinal chemistry. Not only pharmacophoric elements are essential for the design and activity of such compounds, but the type and length of linkers used to connect them are also crucial. In the present contribution, we describe compound 1 in which a typical opioid peptide sequence is combined with a fragment characteristic for neurokinin-1 receptor (NK1R) antagonists through a hydrazone bridge. The compound has a high affinity for μ- and δ-opioid receptors (IC50= 12.7 and 74.0 nM, respectively) and a weak affinity for the NK1R. Molecular modeling and structural considerations explain the observed activities. In in vivo test, intrathecal and intravenous administrations of 1 exhibited a strong analgesic effect, which indicates potential BBB penetration. This letter brings an exemplary application of the hydrazone linker for fast, facile, and successful preparation of chimeric compounds.
Keywords: Bifunctional ligands; NK1 antagonists; hydrazone linker; opioid agonists.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
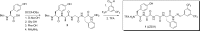
Similar articles
-
Dual Alleviation of Acute and Neuropathic Pain by Fused Opioid Agonist-Neurokinin 1 Antagonist Peptidomimetics.ACS Med Chem Lett. 2015 Oct 31;6(12):1209-14. doi: 10.1021/acsmedchemlett.5b00359. eCollection 2015 Dec 10. ACS Med Chem Lett. 2015. PMID: 26713106 Free PMC article.
-
Analgesic Properties of Opioid/NK1 Multitarget Ligands with Distinct in Vitro Profiles in Naive and Chronic Constriction Injury Mice.ACS Chem Neurosci. 2017 Oct 18;8(10):2315-2324. doi: 10.1021/acschemneuro.7b00226. Epub 2017 Jul 26. ACS Chem Neurosci. 2017. PMID: 28699350 Free PMC article.
-
Two nonpeptide tachykinin antagonists act through epitopes on corresponding segments of the NK1 and NK2 receptors.Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6194-8. doi: 10.1073/pnas.90.13.6194. Proc Natl Acad Sci U S A. 1993. PMID: 7687062 Free PMC article.
-
The Significance of NK1 Receptor Ligands and Their Application in Targeted Radionuclide Tumour Therapy.Pharmaceutics. 2019 Sep 1;11(9):443. doi: 10.3390/pharmaceutics11090443. Pharmaceutics. 2019. PMID: 31480582 Free PMC article. Review.
-
The tachykinin NK1 receptor. Part I: ligands and mechanisms of cellular activation.Neuropeptides. 1997 Dec;31(6):537-63. doi: 10.1016/s0143-4179(97)90001-9. Neuropeptides. 1997. PMID: 9574822 Review.
Cited by
-
Assembling a Cinnamyl Pharmacophore in the C3-Position of Substituted Isatins via Microwave-Assisted Synthesis: Development of a New Class of Monoamine Oxidase-B Inhibitors for the Treatment of Parkinson's Disease.Molecules. 2023 Aug 21;28(16):6167. doi: 10.3390/molecules28166167. Molecules. 2023. PMID: 37630420 Free PMC article.
-
Antinociceptive and Cytotoxic Activity of Opioid Peptides with Hydrazone and Hydrazide Moieties at the C-Terminus.Molecules. 2020 Jul 28;25(15):3429. doi: 10.3390/molecules25153429. Molecules. 2020. PMID: 32731576 Free PMC article.
-
Peptidomimetics and Their Applications for Opioid Peptide Drug Discovery.Biomolecules. 2022 Sep 5;12(9):1241. doi: 10.3390/biom12091241. Biomolecules. 2022. PMID: 36139079 Free PMC article. Review.
-
Catalyst- and Additive-Free C(sp3)-H Functionalization of (Thio)barbituric Acids via C-5 Dehydrogenative Aza-Coupling Under Ambient Conditions.ACS Omega. 2022 Aug 17;7(34):30051-30063. doi: 10.1021/acsomega.2c03073. eCollection 2022 Aug 30. ACS Omega. 2022. PMID: 36061699 Free PMC article.
-
Fentanyl Family at the Mu-Opioid Receptor: Uniform Assessment of Binding and Computational Analysis.Molecules. 2019 Feb 19;24(4):740. doi: 10.3390/molecules24040740. Molecules. 2019. PMID: 30791394 Free PMC article.
References
-
- Ng G. Y. K.; Coulombe N.; Ethier N.; Hebert T. E.; Sullivan R.; Kargman S.; Chateauneuf A.; Tsukamoto N.; Mcdonald T.; Johnson M. P.; Whiting P.; Liu Q.; Kolakowski L. F.; Evans J. F.; Bonner T. I.; O’Neill G. P. Identification of a GABA B Receptor Subunit, gb2, Required for Functional GABA B Receptor Activity. J. Biol. Chem. 1999, 274, 7607–7610. 10.1074/jbc.274.12.7607. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials

