Simultaneous analyses of N-linked and O-linked glycans of ovarian cancer cells using solid-phase chemoenzymatic method
- PMID: 28100988
- PMCID: PMC5237303
- DOI: 10.1186/s12014-017-9137-1
Simultaneous analyses of N-linked and O-linked glycans of ovarian cancer cells using solid-phase chemoenzymatic method
Abstract
Background: Glycans play critical roles in a number of biological activities. Two common types of glycans, N-linked and O-linked, have been extensively analyzed in the last decades. N-glycans are typically released from glycoproteins by enzymes, while O-glycans are released from glycoproteins by chemical methods. It is important to identify and quantify both N- and O-linked glycans of glycoproteins to determine the changes of glycans.
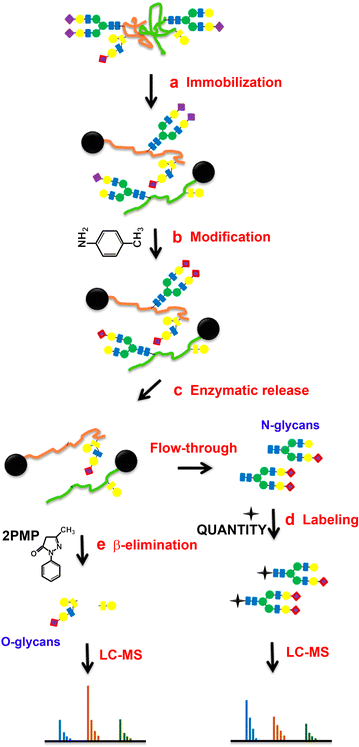
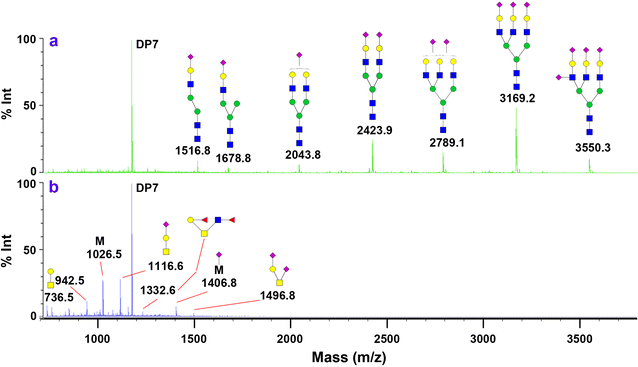
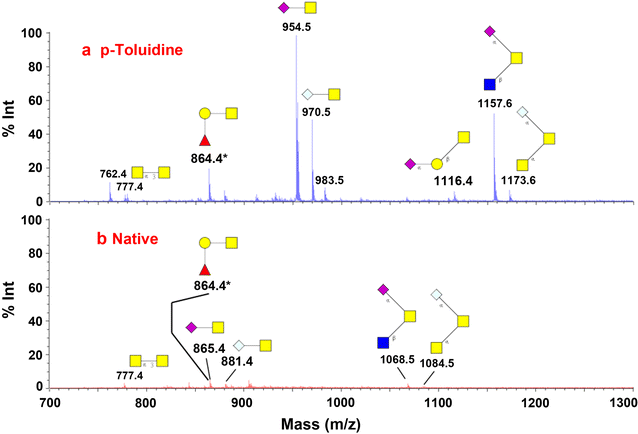
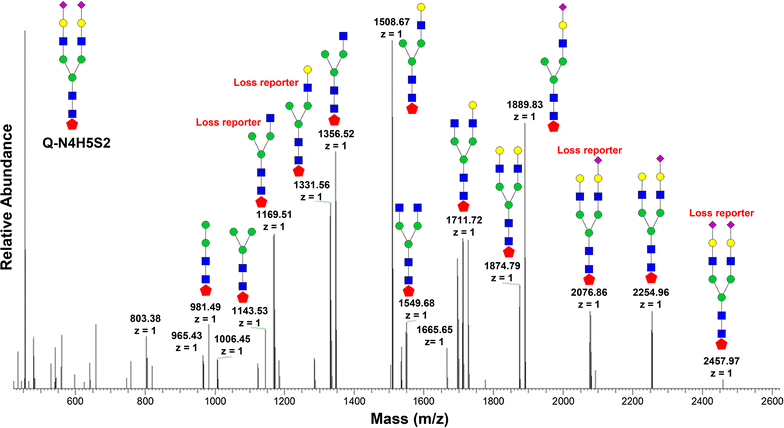
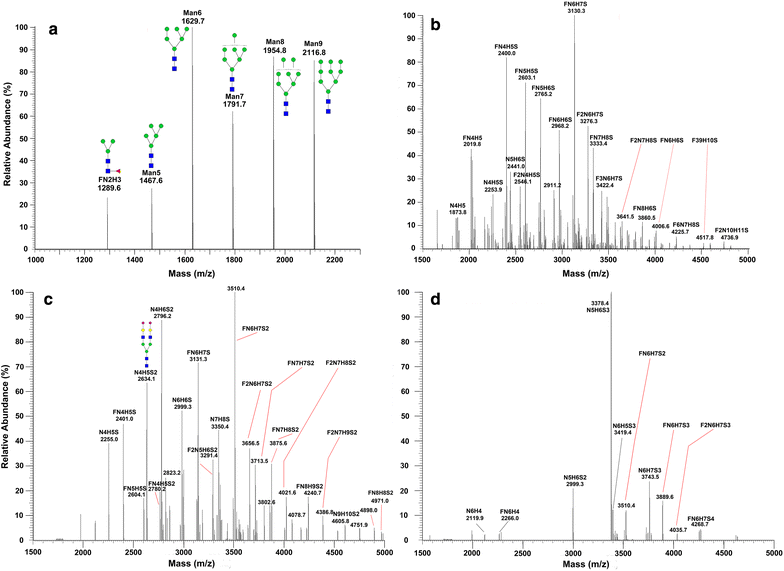
Methods: The effort has been dedicated to study glycans from ovarian cancer cells treated with O-linked glycosylation inhibitor qualitatively and quantitatively. We used a solid-phase chemoenzymatic approach to systematically identify and quantify N-glycans and O-glycans in the ovarian cancer cells. It consists of three steps: (1) immobilization of proteins from cells and derivatization of glycans to protect sialic acids; (2) release of N-glycans by PNGase F and quantification of N-glycans by isobaric tags; (3) release and quantification of O-glycans by β-elimination in the presence of 1-phenyl-3-methyl-5-pyrazolone (PMP).
Results: We used ovarian cancer cell lines to study effect of O-linked glycosylation inhibitor on protein glycosylation. Results suggested that the inhibition of O-linked glycosylation reduced the levels of O-glycans. Interestingly, it appeared to increase N-glycan level in a lower dose of the O-linked glycosylation inhibitor. The sequential release and analyses of N-linked and O-linked glycans using chemoenzymatic approach are a platform for studying N-glycans and O-glycans in complex biological samples.
Conclusion: The solid-phase chemoenzymatic method was used to analyze both N-linked and O-linked glycans sequentially released from the ovarian cancer cells. The biological studies on O-linked glycosylation inhibition indicate the effects of O-glycosylation inhibition to glycan changes in both O-linked and N-linked glycan expression.
Keywords: Chemoenzymatic; Glycomics; Glycoprotein; Solid phase.
Figures
Similar articles
-
Quantitative O-glycomics based on improvement of the one-pot method for nonreductive O-glycan release and simultaneous stable isotope labeling with 1-(d0/d5)phenyl-3-methyl-5-pyrazolone followed by mass spectrometric analysis.J Proteomics. 2017 Jan 6;150:18-30. doi: 10.1016/j.jprot.2016.08.012. Epub 2016 Aug 29. J Proteomics. 2017. PMID: 27585995
-
Simultaneous quantification of N- and O-glycans using a solid-phase method.Nat Protoc. 2017 Jun;12(6):1229-1244. doi: 10.1038/nprot.2017.034. Epub 2017 May 18. Nat Protoc. 2017. PMID: 28518173 Free PMC article.
-
Glycomic analysis of glycans released from glycoproteins using chemical immobilization and mass spectrometry.Curr Protoc Chem Biol. 2014 Sep 9;6(3):191-208. doi: 10.1002/9780470559277.ch140085. Curr Protoc Chem Biol. 2014. PMID: 25205566 Free PMC article.
-
Chemoenzymatic method for glycomics: Isolation, identification, and quantitation.Proteomics. 2016 Jan;16(2):241-56. doi: 10.1002/pmic.201500266. Epub 2015 Nov 6. Proteomics. 2016. PMID: 26390280 Free PMC article. Review.
-
Recent progress in chemoenzymatic synthesis of human glycans.Org Biomol Chem. 2024 Oct 2;22(38):7767-7785. doi: 10.1039/d4ob01006j. Org Biomol Chem. 2024. PMID: 39246045 Review.
Cited by
-
Mass Spectrometry Imaging for Glycome in the Brain.Front Neuroanat. 2021 Jul 29;15:711955. doi: 10.3389/fnana.2021.711955. eCollection 2021. Front Neuroanat. 2021. PMID: 34393728 Free PMC article. Review.
-
Overexpression of Exportin-5 Overrides the Inhibitory Effect of miRNAs Regulation Control and Stabilize Proteins via Posttranslation Modifications in Prostate Cancer.Neoplasia. 2017 Oct;19(10):817-829. doi: 10.1016/j.neo.2017.07.008. Epub 2017 Sep 4. Neoplasia. 2017. PMID: 28881308 Free PMC article.
-
Mass Spectrometry Approaches to Glycomic and Glycoproteomic Analyses.Chem Rev. 2018 Sep 12;118(17):7886-7930. doi: 10.1021/acs.chemrev.7b00732. Epub 2018 Mar 19. Chem Rev. 2018. PMID: 29553244 Free PMC article. Review.
-
A MATLAB-based app to improve LC-MS/MS data analysis for N-linked glycan peak identification.BMC Bioinformatics. 2023 Jun 17;24(1):259. doi: 10.1186/s12859-023-05346-5. BMC Bioinformatics. 2023. PMID: 37330473 Free PMC article.
-
Site-specific glycosylation of SARS-CoV-2: Big challenges in mass spectrometry analysis.Proteomics. 2022 Aug;22(15-16):e2100322. doi: 10.1002/pmic.202100322. Epub 2022 Jun 22. Proteomics. 2022. PMID: 35700310 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
