Neurotoxic reactive astrocytes are induced by activated microglia
- PMID: 28099414
- PMCID: PMC5404890
- DOI: 10.1038/nature21029
Neurotoxic reactive astrocytes are induced by activated microglia
Abstract
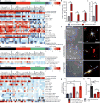
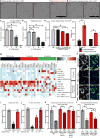
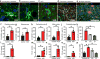
Reactive astrocytes are strongly induced by central nervous system (CNS) injury and disease, but their role is poorly understood. Here we show that a subtype of reactive astrocytes, which we termed A1, is induced by classically activated neuroinflammatory microglia. We show that activated microglia induce A1 astrocytes by secreting Il-1α, TNF and C1q, and that these cytokines together are necessary and sufficient to induce A1 astrocytes. A1 astrocytes lose the ability to promote neuronal survival, outgrowth, synaptogenesis and phagocytosis, and induce the death of neurons and oligodendrocytes. Death of axotomized CNS neurons in vivo is prevented when the formation of A1 astrocytes is blocked. Finally, we show that A1 astrocytes are abundant in various human neurodegenerative diseases including Alzheimer's, Huntington's and Parkinson's disease, amyotrophic lateral sclerosis and multiple sclerosis. Taken together these findings help to explain why CNS neurons die after axotomy, strongly suggest that A1 astrocytes contribute to the death of neurons and oligodendrocytes in neurodegenerative disorders, and provide opportunities for the development of new treatments for these diseases.
Figures




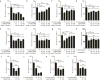
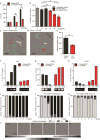
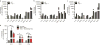
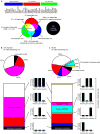



Comment in
-
Glia: A toxic reaction.Nat Rev Neurosci. 2017 Mar;18(3):130. doi: 10.1038/nrn.2017.13. Epub 2017 Feb 2. Nat Rev Neurosci. 2017. PMID: 28148955 No abstract available.
-
Neuroimmunology: Microglia-induced reactive astrocytes - toxic players in neurological disease?Nat Rev Neurol. 2017 Mar;13(3):127. doi: 10.1038/nrneurol.2017.17. Epub 2017 Feb 3. Nat Rev Neurol. 2017. PMID: 28155893 No abstract available.
Similar articles
-
MFG-E8 reverses microglial-induced neurotoxic astrocyte (A1) via NF-κB and PI3K-Akt pathways.J Cell Physiol. 2018 Jan;234(1):904-914. doi: 10.1002/jcp.26918. Epub 2018 Aug 4. J Cell Physiol. 2018. PMID: 30076715
-
The role of reactive astrocytes in neurotoxicity induced by ultrafine particulate matter.Sci Total Environ. 2023 Apr 1;867:161416. doi: 10.1016/j.scitotenv.2023.161416. Epub 2023 Jan 5. Sci Total Environ. 2023. PMID: 36621481
-
Normal aging induces A1-like astrocyte reactivity.Proc Natl Acad Sci U S A. 2018 Feb 20;115(8):E1896-E1905. doi: 10.1073/pnas.1800165115. Epub 2018 Feb 7. Proc Natl Acad Sci U S A. 2018. PMID: 29437957 Free PMC article.
-
Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes.Transl Neurodegener. 2020 Nov 26;9(1):42. doi: 10.1186/s40035-020-00221-2. Transl Neurodegener. 2020. PMID: 33239064 Free PMC article. Review.
-
Roles of neuropathology-associated reactive astrocytes: a systematic review.Acta Neuropathol Commun. 2023 Mar 13;11(1):42. doi: 10.1186/s40478-023-01526-9. Acta Neuropathol Commun. 2023. PMID: 36915214 Free PMC article. Review.
Cited by
-
The Role of Astrocytes and Alpha-Synuclein in Parkinson's Disease: A Review.NeuroSci. 2024 Mar 8;5(1):71-86. doi: 10.3390/neurosci5010005. eCollection 2024 Mar. NeuroSci. 2024. PMID: 39483813 Free PMC article. Review.
-
Targeting astrocytes polarization after spinal cord injury: a promising direction.Front Cell Neurosci. 2024 Oct 16;18:1478741. doi: 10.3389/fncel.2024.1478741. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39479524 Free PMC article. Review.
-
Neuromodulation of Glial Function During Neurodegeneration.Front Cell Neurosci. 2020 Aug 21;14:278. doi: 10.3389/fncel.2020.00278. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32973460 Free PMC article.
-
Astrocyte Responses to Complement Peptide C3a are Highly Context-Dependent.Neurochem Res. 2023 Apr;48(4):1233-1241. doi: 10.1007/s11064-022-03743-5. Epub 2022 Sep 12. Neurochem Res. 2023. PMID: 36097103 Free PMC article.
-
Exciting Complexity: The Role of Motor Circuit Elements in ALS Pathophysiology.Front Neurosci. 2020 Jun 17;14:573. doi: 10.3389/fnins.2020.00573. eCollection 2020. Front Neurosci. 2020. PMID: 32625051 Free PMC article. Review.
References
-
- Liddelow S, Barres B. SnapShot: Astrocytes in Health and Disease. Cell. 2015;162:1170–1170 e1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

