Enhanced neuroinflammation mediated by DNA methylation of the glucocorticoid receptor triggers cognitive dysfunction after sevoflurane anesthesia in adult rats subjected to maternal separation during the neonatal period
- PMID: 28086911
- PMCID: PMC5234142
- DOI: 10.1186/s12974-016-0782-5
Enhanced neuroinflammation mediated by DNA methylation of the glucocorticoid receptor triggers cognitive dysfunction after sevoflurane anesthesia in adult rats subjected to maternal separation during the neonatal period
Abstract
Background: Mounting evidence indicates that children who experience abuse and neglect are prone to chronic diseases and premature mortality later in life. One mechanistic hypothesis for this phenomenon is that early life adversity alters the expression or functioning of the glucocorticoid receptor (GR) throughout the course of life and thereby increases sensitivity to inflammatory stimulation. An exaggerated pro-inflammatory response is generally considered to be a key cause of postoperative cognitive dysfunction (POCD). The aim of this study was to examine the effects of early life adversity on cognitive function and neuroinflammation after sevoflurane anesthesia in adult rats and to determine whether such effects are associated with the epigenetic regulation of GR.
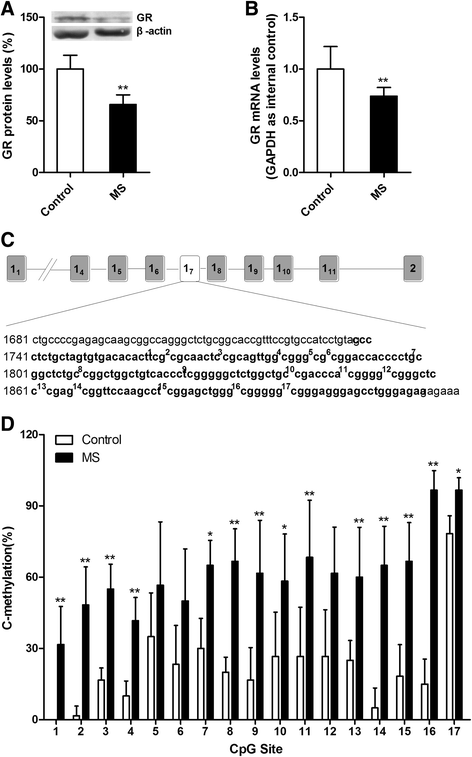
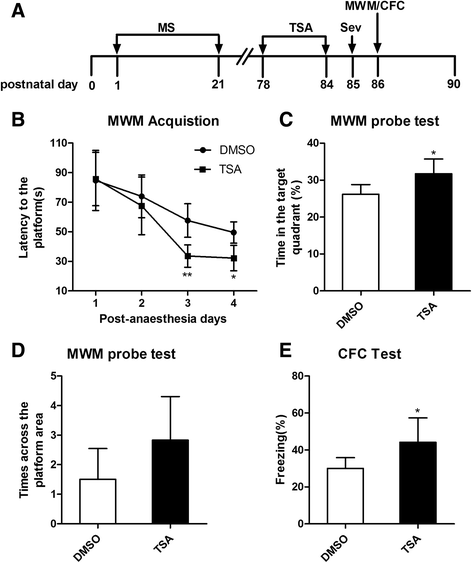
Methods: Wistar rat pups were repeatedly subjected to infant maternal separation (early life stress) from postnatal days 2-21. In adulthood, their behavior and the signaling of hippocampal pro-inflammatory factors and nuclear factor-kappa B (NF-κB) after sevoflurane anesthesia were evaluated. We also examined the effects of maternal separation (MS) on the expression of GR and the DNA methylation status of the promoter region of exon 17 of GR and whether behavioral changes and neuroinflammation after anesthesia were reversible when the expression of GR was increased by altering DNA methylation.
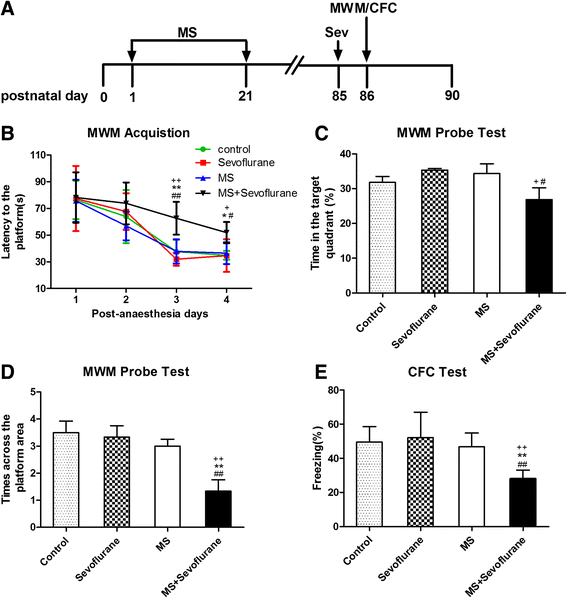
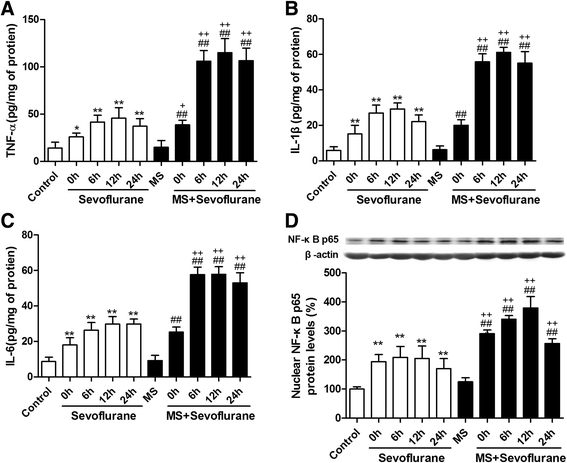
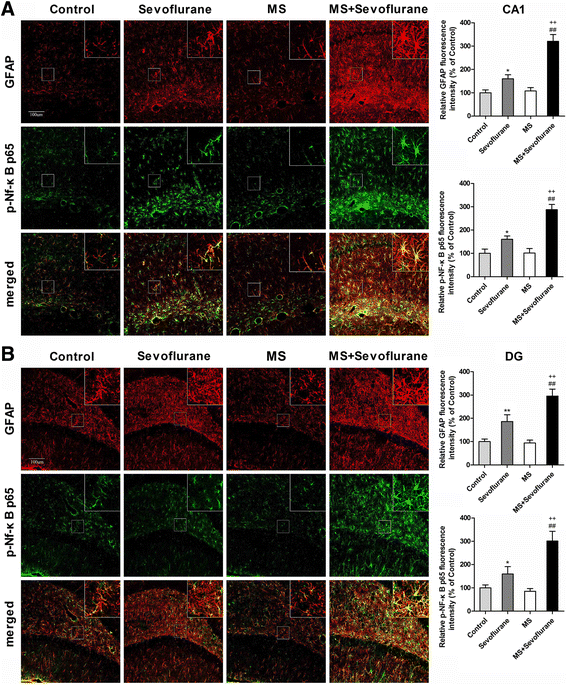
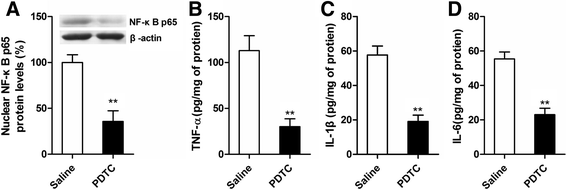
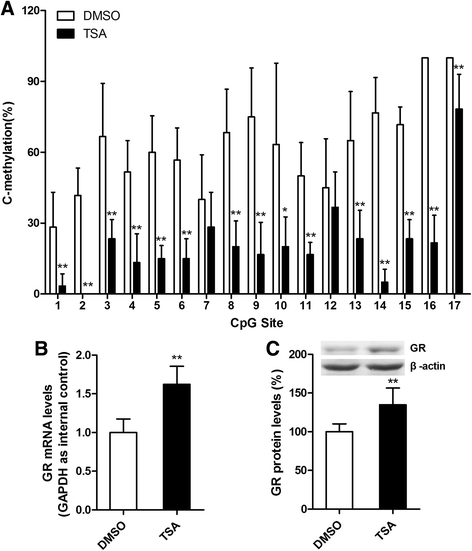
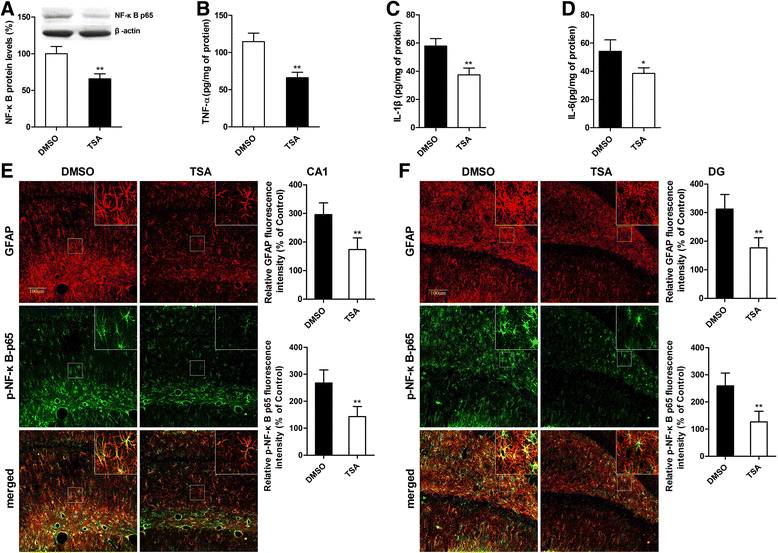
Results: MS induced cognitive decline after sevoflurane inhalation in the Morris water maze and context fear conditioning tests and enhanced the release of cytokines and the activation of astrocyte intracellular NF-κB signaling induced by sevoflurane in the hippocampus of adult rats. Blocking NF-κB signaling by pyrrolidine dithiocarbamate (PDTC) inhibited the release of cytokines. MS also reduced the expression of GR and upregulated the methylation levels of the promoter region of GR exon 17, and such effects were reversed by treatment with the histone deacetylase inhibitor trichostatin A (TSA) in adult rats. Moreover, TSA treatment in adult MS rats inhibited the overactivation of astrocyte intracellular NF-κB signaling and the release of cytokines and alleviated cognitive dysfunction after sevoflurane anesthesia.
Conclusions: Early life stress induces cognitive dysfunction after sevoflurane anesthesia, perhaps due to the aberrant methylation of the GR gene promoter, which reduces the expression of the GR gene and facilitates exaggerated inflammatory responses.
Keywords: Cognitive dysfunction; DNA methylation; Glucocorticoid receptor; Neuroinflammation; Pro-inflammatory factors; Sevoflurane.
Figures
Similar articles
-
Hypermethylation of Hippocampal Synaptic Plasticity-Related genes is Involved in Neonatal Sevoflurane Exposure-Induced Cognitive Impairments in Rats.Neurotox Res. 2016 Feb;29(2):243-55. doi: 10.1007/s12640-015-9585-1. Epub 2015 Dec 17. Neurotox Res. 2016. PMID: 26678494
-
Hydrogen gas attenuates sevoflurane neurotoxicity through inhibiting nuclear factor κ-light-chain-enhancer of activated B cells signaling and proinflammatory cytokine release in neonatal rats.Neuroreport. 2017 Dec 6;28(17):1170-1175. doi: 10.1097/WNR.0000000000000899. Neuroreport. 2017. PMID: 28926473
-
IL-17A promotes the neuroinflammation and cognitive function in sevoflurane anesthetized aged rats via activation of NF-κB signaling pathway.BMC Anesthesiol. 2018 Oct 20;18(1):147. doi: 10.1186/s12871-018-0607-4. BMC Anesthesiol. 2018. PMID: 30342469 Free PMC article.
-
Effects of the Social Environment and Stress on Glucocorticoid Receptor Gene Methylation: A Systematic Review.Biol Psychiatry. 2016 Jan 15;79(2):87-96. doi: 10.1016/j.biopsych.2014.11.022. Epub 2014 Dec 13. Biol Psychiatry. 2016. PMID: 25687413 Free PMC article. Review.
-
Cytokines and glucocorticoid receptor signaling. Relevance to major depression.Ann N Y Acad Sci. 2009 Oct;1179:86-105. doi: 10.1111/j.1749-6632.2009.04984.x. Ann N Y Acad Sci. 2009. PMID: 19906234 Free PMC article. Review.
Cited by
-
Effect of General Anesthetic Agents on Microglia.Aging Dis. 2024 May 7;15(3):1308-1328. doi: 10.14336/AD.2023.1108. Aging Dis. 2024. PMID: 37962460 Free PMC article. Review.
-
Perioperative neurocognitive dysfunction: thinking from the gut?Aging (Albany NY). 2020 Aug 15;12(15):15797-15817. doi: 10.18632/aging.103738. Epub 2020 Aug 15. Aging (Albany NY). 2020. PMID: 32805716 Free PMC article.
-
Egr2 contributes to age-dependent vulnerability to sevoflurane-induced cognitive deficits in mice.Acta Pharmacol Sin. 2022 Nov;43(11):2828-2840. doi: 10.1038/s41401-022-00915-5. Epub 2022 May 16. Acta Pharmacol Sin. 2022. PMID: 35577909 Free PMC article.
-
Smad7 in the hippocampus contributes to memory impairment in aged mice after anesthesia and surgery.J Neuroinflammation. 2023 Jul 28;20(1):175. doi: 10.1186/s12974-023-02849-z. J Neuroinflammation. 2023. PMID: 37507781 Free PMC article.
-
Major surgery induces acute changes in measured DNA methylation associated with immune response pathways.Sci Rep. 2020 Apr 1;10(1):5743. doi: 10.1038/s41598-020-62262-x. Sci Rep. 2020. PMID: 32238836 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

