Multi-level suppression of receptor-PI3K-mTORC1 by fatty acid synthase inhibitors is crucial for their efficacy against ovarian cancer cells
- PMID: 28086243
- PMCID: PMC5355289
- DOI: 10.18632/oncotarget.14591
Multi-level suppression of receptor-PI3K-mTORC1 by fatty acid synthase inhibitors is crucial for their efficacy against ovarian cancer cells
Abstract
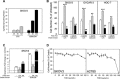
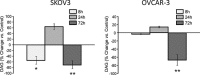
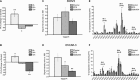
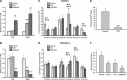
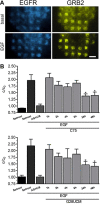
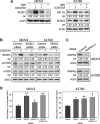
Receptor-PI3K-mTORC1 signaling and fatty acid synthase (FASN)-regulated lipid biosynthesis harbor numerous drug targets and are molecularly connected. We hypothesize that unraveling the mechanisms of pathway cross-talk will be useful for designing novel co-targeting strategies for ovarian cancer (OC). The impact of receptor-PI3K-mTORC1 onto FASN is already well-characterized. However, reverse actions-from FASN towards receptor-PI3K-mTORC1-are still elusive. We show that FASN-blockade impairs receptor-PI3K-mTORC1 signaling at multiple levels. Thin-layer chromatography and MALDI-MS/MS reveals that FASN-inhibitors (C75, G28UCM) augment polyunsaturated fatty acids and diminish signaling lipids diacylglycerol (DAG) and phosphatidylinositol 3,4,5-trisphosphate (PIP3) in OC cells (SKOV3, OVCAR-3, A2780, HOC-7). Western blotting and micropatterning demonstrate that FASN-blockers impair phosphorylation/expression of EGF-receptor/ERBB/HER and decrease GRB2-EGF-receptor recruitment leading to PI3K-AKT suppression. FASN-inhibitors activate stress response-genes HIF-1α-REDD1 (RTP801/DIG2/DDIT4) and AMPKα causing mTORC1- and S6-repression. We conclude that FASN-inhibitor-mediated blockade of receptor-PI3K-mTORC1 occurs due to a number of distinct but cooperating processes. Moreover, decrease of PI3K-mTORC1 abolishes cross-repression of MEK-ERK causing ERK activation. Consequently, the MEK-inhibitor selumetinib/AZD6244, in contrast to the PI3K/mTOR-inhibitor dactolisib/NVP-BEZ235, increases growth inhibition when given together with a FASN-blocker. We are the first to provide deep insight on how FASN-inhibition blocks ERBB-PI3K-mTORC1 activity at multiple molecular levels. Moreover, our data encourage therapeutic approaches using FASN-antagonists together with MEK-ERK-inhibitors.
Keywords: AMPK; REDD1; fatty acid synthase (FASN); lipids; mTORC1.
Conflict of interest statement
The authors have no potential conflicts of interest.
Figures


Similar articles
-
Blockade of fatty acid synthase induces ubiquitination and degradation of phosphoinositide-3-kinase signaling proteins in ovarian cancer.Mol Cancer Res. 2011 Dec;9(12):1767-79. doi: 10.1158/1541-7786.MCR-10-0467. Epub 2011 Oct 4. Mol Cancer Res. 2011. PMID: 21970855
-
Fatty acid synthase inhibition engages a novel caspase-2 regulatory mechanism to induce ovarian cancer cell death.Oncogene. 2015 Jun;34(25):3264-72. doi: 10.1038/onc.2014.271. Epub 2014 Aug 25. Oncogene. 2015. PMID: 25151963 Free PMC article.
-
Simultaneous perturbation of the MAPK and the PI3K/mTOR pathways does not lead to increased radiosensitization.Radiat Oncol. 2015 Oct 24;10:214. doi: 10.1186/s13014-015-0514-5. Radiat Oncol. 2015. PMID: 26498922 Free PMC article.
-
Targeting fatty acid synthase in breast and endometrial cancer: An alternative to selective estrogen receptor modulators?Endocrinology. 2006 Sep;147(9):4056-66. doi: 10.1210/en.2006-0486. Epub 2006 Jun 29. Endocrinology. 2006. PMID: 16809439 Review.
-
The PI3K/AKT/mTOR pathway as a therapeutic target in ovarian cancer.Gynecol Oncol. 2015 Apr;137(1):173-9. doi: 10.1016/j.ygyno.2015.02.003. Epub 2015 Feb 10. Gynecol Oncol. 2015. PMID: 25677064 Review.
Cited by
-
Metabolic reprogramming of the ovarian cancer microenvironment in the development of antiangiogenic resistance.Acta Biochim Biophys Sin (Shanghai). 2023 Apr 6;55(6):938-947. doi: 10.3724/abbs.2023046. Acta Biochim Biophys Sin (Shanghai). 2023. PMID: 37021977 Free PMC article. Review.
-
Deregulation of Lipid Metabolism: The Critical Factors in Ovarian Cancer.Front Oncol. 2020 Oct 19;10:593017. doi: 10.3389/fonc.2020.593017. eCollection 2020. Front Oncol. 2020. PMID: 33194756 Free PMC article. Review.
-
Metformin alleviates inflammation through suppressing FASN-dependent palmitoylation of Akt.Cell Death Dis. 2021 Oct 12;12(10):934. doi: 10.1038/s41419-021-04235-0. Cell Death Dis. 2021. PMID: 34642298 Free PMC article.
-
Highly Modular Protein Micropatterning Sheds Light on the Role of Clathrin-Mediated Endocytosis for the Quantitative Analysis of Protein-Protein Interactions in Live Cells.Biomolecules. 2020 Apr 2;10(4):540. doi: 10.3390/biom10040540. Biomolecules. 2020. PMID: 32252486 Free PMC article.
-
Today's cancer research and treatment - highly sophisticated and molecularly targeted, yet firmly bolstered in the classical theories.J Appl Biomed. 2024 Sep;22(3):123-128. doi: 10.32725/jab.2024.016. Epub 2024 Sep 2. J Appl Biomed. 2024. PMID: 39434508 Review.
References
-
- Banks E, Beral V, Reeves G. The epidemiology of epithelial ovarian cancer: a review. Int J Gynecol Cancer. 1997;7:425–438.
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. - PubMed
-
- SEER Stat Fact Sheets: Ovary Cancer [online]. Available from: http://seer.cancer.gov/statfacts/html/ovary.html Retrieved on January 19, 2016.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

