MiR-26 enhances chemosensitivity and promotes apoptosis of hepatocellular carcinoma cells through inhibiting autophagy
- PMID: 28079894
- PMCID: PMC5386370
- DOI: 10.1038/cddis.2016.461
MiR-26 enhances chemosensitivity and promotes apoptosis of hepatocellular carcinoma cells through inhibiting autophagy
Abstract
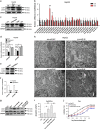
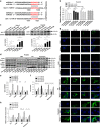
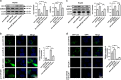
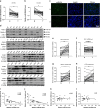
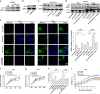
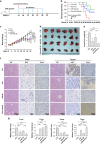
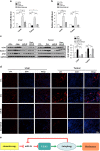
Hepatocellular carcinoma (HCC) generally possesses a high resistance to chemotherapy. Given that autophagy is an important factor promoting tumor chemoresistance and HCC express low level of miR-26, we aim to investigate the functional role of miR-26 in autophagy-mediated chemoresistance of HCC. We found that chemotherapeutic drug doxorubicin (Dox) induced autophagy but decreased the level of miR-26a/b in HCC cells. Activating autophagy using rapamycin can directly downregulate the level of miR-26a/b in HCC cells. In turn, restoring the expression of miR-26a/b inhibited autophagy induced by Dox and promoted apoptosis in HCC cells. Further mechanistic study identified that miR-26a and miR-26b target ULK1, a critical initiator of autophagy, at post-transcriptional level. Results from 30 cases of patients with HCC also showed that tumor cellular levels of miR-26a and miR-26b are significantly downregulated as compared with the corresponding control tissues and negatively correlated with the protein level of ULK1 but are not correlated to the mRNA level of ULK1. Gain- and loss-of-function assay confirmed that miR-26a/b inhibited autophagic flux at the initial stage through targeting ULK1. Overexpression of miR-26a/b enhanced the sensitivity of HCC cells to Dox and promoted apoptosis via inhibiting autophagy in vitro. Using xenograft models in nude mice, we confirmed that miR-26a/b, via inhibiting autophagy, promoted apoptosis and sensitized hepatomas to Dox treatment in vivo. Our findings demonstrate for the first time that miR-26a/b can promote apoptosis and sensitize HCC to chemotherapy via suppressing the expression of autophagy initiator ULK1, and provide the reduction of miR-26a/b in HCC as a novel mechanism of tumor chemoresistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
MiR-26a-5p Inhibits Cell Proliferation and Enhances Doxorubicin Sensitivity in HCC Cells via Targeting AURKA.Technol Cancer Res Treat. 2019 Jan 1;18:1533033819851833. doi: 10.1177/1533033819851833. Technol Cancer Res Treat. 2019. PMID: 31570091 Free PMC article.
-
MiR-21 mediates sorafenib resistance of hepatocellular carcinoma cells by inhibiting autophagy via the PTEN/Akt pathway.Oncotarget. 2015 Oct 6;6(30):28867-81. doi: 10.18632/oncotarget.4814. Oncotarget. 2015. PMID: 26311740 Free PMC article.
-
MicroRNA-383 inhibits doxorubicin resistance in hepatocellular carcinoma by targeting eukaryotic translation initiation factor 5A2.J Cell Mol Med. 2019 Nov;23(11):7190-7199. doi: 10.1111/jcmm.14197. Epub 2019 Feb 23. J Cell Mol Med. 2019. PMID: 30801960 Free PMC article.
-
Human hepatocellular carcinoma: Protection by melatonin.J Cell Physiol. 2018 Oct;233(10):6486-6508. doi: 10.1002/jcp.26586. Epub 2018 Apr 19. J Cell Physiol. 2018. PMID: 29672851 Review.
-
Secretory Clusterin: A Promising Target for Chemoresistance of Hepatocellular Carcinoma.Mini Rev Med Chem. 2020;20(12):1153-1165. doi: 10.2174/1389557520666200331072122. Mini Rev Med Chem. 2020. PMID: 32228422 Review.
Cited by
-
miRNAs Involved in Esophageal Carcinogenesis and miRNA-Related Therapeutic Perspectives in Esophageal Carcinoma.Int J Mol Sci. 2021 Mar 31;22(7):3640. doi: 10.3390/ijms22073640. Int J Mol Sci. 2021. PMID: 33807389 Free PMC article. Review.
-
Regulation of KIR3DL3 Expression via Mirna.Genes (Basel). 2019 Aug 9;10(8):603. doi: 10.3390/genes10080603. Genes (Basel). 2019. PMID: 31405037 Free PMC article.
-
MicroRNA-Based Combinatorial Cancer Therapy: Effects of MicroRNAs on the Efficacy of Anti-Cancer Therapies.Cells. 2019 Dec 20;9(1):29. doi: 10.3390/cells9010029. Cells. 2019. PMID: 31861937 Free PMC article. Review.
-
Autophagy, Metabolism, and Alcohol-Related Liver Disease: Novel Modulators and Functions.Int J Mol Sci. 2019 Oct 11;20(20):5029. doi: 10.3390/ijms20205029. Int J Mol Sci. 2019. PMID: 31614437 Free PMC article. Review.
-
Non-Coding RNAs: Regulating Disease Progression and Therapy Resistance in Hepatocellular Carcinoma.Cancers (Basel). 2020 May 15;12(5):1243. doi: 10.3390/cancers12051243. Cancers (Basel). 2020. PMID: 32429062 Free PMC article. Review.
References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global Cancer Statistics. CA-Cancer J Clin 2011; 61: 69–90. - PubMed
-
- Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: Efficacy and doxorubicin pharmacokinetics. J Hepatol 2007; 46: 474–481. - PubMed
-
- Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med 2002; 53: 615–627. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

