Neonatal Transplantation Confers Maturation of PSC-Derived Cardiomyocytes Conducive to Modeling Cardiomyopathy
- PMID: 28076798
- PMCID: PMC5232412
- DOI: 10.1016/j.celrep.2016.12.040
Neonatal Transplantation Confers Maturation of PSC-Derived Cardiomyocytes Conducive to Modeling Cardiomyopathy
Abstract
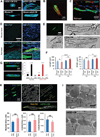
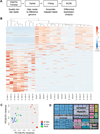
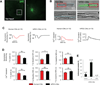
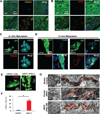
Pluripotent stem cells (PSCs) offer unprecedented opportunities for disease modeling and personalized medicine. However, PSC-derived cells exhibit fetal-like characteristics and remain immature in a dish. This has emerged as a major obstacle for their application for late-onset diseases. We previously showed that there is a neonatal arrest of long-term cultured PSC-derived cardiomyocytes (PSC-CMs). Here, we demonstrate that PSC-CMs mature into adult CMs when transplanted into neonatal hearts. PSC-CMs became similar to adult CMs in morphology, structure, and function within a month of transplantation into rats. The similarity was further supported by single-cell RNA-sequencing analysis. Moreover, this in vivo maturation allowed patient-derived PSC-CMs to reveal the disease phenotype of arrhythmogenic right ventricular cardiomyopathy, which manifests predominantly in adults. This study lays a foundation for understanding human CM maturation and pathogenesis and can be instrumental in PSC-based modeling of adult heart diseases.
Keywords: ARVC; T-tubule; calcium transient; cardiac progenitor; cardiomyocyte; cardiomyopathy; disease modeling; iPS; maturation; neonatal; sarcomere shortening.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
MicroRNA-mediated maturation of human pluripotent stem cell-derived cardiomyocytes: Towards a better model for cardiotoxicity?Food Chem Toxicol. 2016 Dec;98(Pt A):17-24. doi: 10.1016/j.fct.2016.05.025. Epub 2016 Jun 3. Food Chem Toxicol. 2016. PMID: 27265266 Review.
-
Use of a neonatal rat system as a bioincubator to generate adult-like mature cardiomyocytes from human and mouse pluripotent stem cells.Nat Protoc. 2017 Oct;12(10):2097-2109. doi: 10.1038/nprot.2017.089. Epub 2017 Sep 7. Nat Protoc. 2017. PMID: 28880277 Free PMC article.
-
Sarcomere Shortening of Pluripotent Stem Cell-Derived Cardiomyocytes using Fluorescent-Tagged Sarcomere Proteins.J Vis Exp. 2021 Mar 3;(169). doi: 10.3791/62129. J Vis Exp. 2021. PMID: 33749676
-
In Vivo Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes in Neonatal and Adult Rat Hearts.Stem Cell Reports. 2017 Feb 14;8(2):278-289. doi: 10.1016/j.stemcr.2016.10.009. Epub 2017 Jan 5. Stem Cell Reports. 2017. PMID: 28065644 Free PMC article.
-
Pluripotent Stem Cell-Derived Cardiomyocyte Transplantation for Heart Disease Treatment.Curr Cardiol Rep. 2019 Jun 21;21(8):73. doi: 10.1007/s11886-019-1171-3. Curr Cardiol Rep. 2019. PMID: 31228011 Review.
Cited by
-
Propagation of human prostate tissue from induced pluripotent stem cells.Stem Cells Transl Med. 2020 Jul;9(7):734-745. doi: 10.1002/sctm.19-0286. Epub 2020 Mar 14. Stem Cells Transl Med. 2020. PMID: 32170918 Free PMC article.
-
The developmental stage of the hematopoietic niche regulates lineage in MLL-rearranged leukemia.J Exp Med. 2019 Mar 4;216(3):527-538. doi: 10.1084/jem.20181765. Epub 2019 Feb 6. J Exp Med. 2019. PMID: 30728174 Free PMC article.
-
Mesendodermal cells fail to contribute to heart formation following blastocyst injection.bioRxiv [Preprint]. 2024 May 23:2024.05.22.595392. doi: 10.1101/2024.05.22.595392. bioRxiv. 2024. PMID: 38826381 Free PMC article. Preprint.
-
Stem Cell Differentiation is Regulated by Extracellular Matrix Mechanics.Physiology (Bethesda). 2018 Jan 1;33(1):16-25. doi: 10.1152/physiol.00026.2017. Physiology (Bethesda). 2018. PMID: 29212889 Free PMC article. Review.
-
Metabolic changes of human induced pluripotent stem cell-derived cardiomyocytes and teratomas after transplantation.iScience. 2024 Oct 23;27(11):111234. doi: 10.1016/j.isci.2024.111234. eCollection 2024 Nov 15. iScience. 2024. PMID: 39569381 Free PMC article.
References
-
- Bassani RA, Bers DM. Na-Ca exchange is required for rest-decay but not for rest-potentiation of twitches in rabbit and rat ventricular myocytes. J Mol Cell Cardiol. 1994;26:1335–1347. - PubMed
-
- Basso C, Czarnowska E, Della Barbera M, Bauce B, Beffagna G, Wlodarska EK, Pilichou K, Ramondo A, Lorenzon A, Wozniek O, et al. Ultrastructural evidence of intercalated disc remodelling in arrhythmogenic right ventricular cardiomyopathy: an electron microscopy investigation on endomyocardial biopsies. Eur Heart J. 2006;27:1847–1854. - PubMed
-
- Calkins H, Marcus F. Arrhythmogenic right ventricular cardiomyopathy/dysplasia: an update. Curr Cardiol Rep. 2008;10:367–375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

