Pathology of Neurodegenerative Diseases
- PMID: 28062563
- PMCID: PMC5495060
- DOI: 10.1101/cshperspect.a028035
Pathology of Neurodegenerative Diseases
Abstract
Neurodegenerative disorders are characterized by progressive loss of selectively vulnerable populations of neurons, which contrasts with select static neuronal loss because of metabolic or toxic disorders. Neurodegenerative diseases can be classified according to primary clinical features (e.g., dementia, parkinsonism, or motor neuron disease), anatomic distribution of neurodegeneration (e.g., frontotemporal degenerations, extrapyramidal disorders, or spinocerebellar degenerations), or principal molecular abnormality. The most common neurodegenerative disorders are amyloidoses, tauopathies, α-synucleinopathies, and TDP-43 proteinopathies. The protein abnormalities in these disorders have abnormal conformational properties. Growing experimental evidence suggests that abnormal protein conformers may spread from cell to cell along anatomically connected pathways, which may in part explain the specific anatomical patterns observed at autopsy. In this review, we detail the human pathology of select neurodegenerative disorders, focusing on their main protein aggregates.
Copyright © 2017 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures



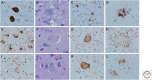
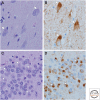
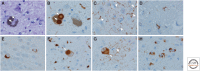
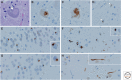
Similar articles
-
Neurodegenerative Disorders of Alzheimer, Parkinsonism, Amyotrophic Lateral Sclerosis and Multiple Sclerosis: An Early Diagnostic Approach for Precision Treatment.Metab Brain Dis. 2022 Jan;37(1):67-104. doi: 10.1007/s11011-021-00800-w. Epub 2021 Nov 1. Metab Brain Dis. 2022. PMID: 34719771 Review.
-
Neuropathology of non-Alzheimer degenerative disorders.Int J Clin Exp Pathol. 2009 Aug 25;3(1):1-23. Int J Clin Exp Pathol. 2009. PMID: 19918325 Free PMC article. Review.
-
Effects of curcumin on mitochondria in neurodegenerative diseases.Biofactors. 2020 Jan;46(1):5-20. doi: 10.1002/biof.1566. Epub 2019 Oct 3. Biofactors. 2020. PMID: 31580521 Review.
-
Neuropathological spectrum of synucleinopathies.Mov Disord. 2003 Sep;18 Suppl 6:S2-12. doi: 10.1002/mds.10557. Mov Disord. 2003. PMID: 14502650 Review.
-
[Neuropathology of tauopathies and synucleinopathies, and neuroanatomy of sleep disorders: meeting the challenge].Rev Neurol (Paris). 2003 Nov;159(11 Suppl):6S59-70. Rev Neurol (Paris). 2003. PMID: 14646802 Review. French.
Cited by
-
Carotenoids in Drug Discovery and Medicine: Pathways and Molecular Targets Implicated in Human Diseases.Molecules. 2022 Sep 15;27(18):6005. doi: 10.3390/molecules27186005. Molecules. 2022. PMID: 36144741 Free PMC article. Review.
-
Common Protective Strategies in Neurodegenerative Disease: Focusing on Risk Factors to Target the Cellular Redox System.Oxid Med Cell Longev. 2020 Aug 1;2020:8363245. doi: 10.1155/2020/8363245. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32832006 Free PMC article. Review.
-
Misfolded protein oligomers: mechanisms of formation, cytotoxic effects, and pharmacological approaches against protein misfolding diseases.Mol Neurodegener. 2024 Feb 20;19(1):20. doi: 10.1186/s13024-023-00651-2. Mol Neurodegener. 2024. PMID: 38378578 Free PMC article. Review.
-
Elucidating the Multi-Targeted Role of Nutraceuticals: A Complementary Therapy to Starve Neurodegenerative Diseases.Int J Mol Sci. 2021 Apr 14;22(8):4045. doi: 10.3390/ijms22084045. Int J Mol Sci. 2021. PMID: 33919895 Free PMC article. Review.
-
Sex difference in human diseases: mechanistic insights and clinical implications.Signal Transduct Target Ther. 2024 Sep 10;9(1):238. doi: 10.1038/s41392-024-01929-7. Signal Transduct Target Ther. 2024. PMID: 39256355 Free PMC article. Review.
References
-
- Alafuzoff I, Ince PG, Arzberger T, Al-Sarraj S, Bell J, Bodi I, Bogdanovic N, Bugiani O, Ferrer I, Gelpi E, et al. 2009a. Staging/typing of Lewy body related α-synuclein pathology: A study of the BrainNet Europe Consortium. Acta Neuropathol 117: 635–652. - PubMed
-
- Alafuzoff I, Pikkarainen M, Neumann M, Arzberger T, Al-Sarraj S, Bodi I, Bogdanovic N, Bugiani O, Ferrer I, Gelpi E, et al. 2015. Neuropathological assessments of the pathology in frontotemporal lobar degeneration with TDP43-positive inclusions: An inter-laboratory study by the BrainNet Europe consortium. J Neural Transm (Vienna) 122: 957–972. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
