Plasma membrane wounding and repair in pulmonary diseases
- PMID: 28062486
- PMCID: PMC5374305
- DOI: 10.1152/ajplung.00486.2016
Plasma membrane wounding and repair in pulmonary diseases
Abstract
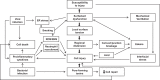
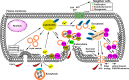
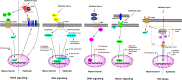
Various pathophysiological conditions such as surfactant dysfunction, mechanical ventilation, inflammation, pathogen products, environmental exposures, and gastric acid aspiration stress lung cells, and the compromise of plasma membranes occurs as a result. The mechanisms necessary for cells to repair plasma membrane defects have been extensively investigated in the last two decades, and some of these key repair mechanisms are also shown to occur following lung cell injury. Because it was theorized that lung wounding and repair are involved in the pathogenesis of acute respiratory distress syndrome (ARDS) and idiopathic pulmonary fibrosis (IPF), in this review, we summarized the experimental evidence of lung cell injury in these two devastating syndromes and discuss relevant genetic, physical, and biological injury mechanisms, as well as mechanisms used by lung cells for cell survival and membrane repair. Finally, we discuss relevant signaling pathways that may be activated by chronic or repeated lung cell injury as an extension of our cell injury and repair focus in this review. We hope that a holistic view of injurious stimuli relevant for ARDS and IPF could lead to updated experimental models. In addition, parallel discussion of membrane repair mechanisms in lung cells and injury-activated signaling pathways would encourage research to bridge gaps in current knowledge. Indeed, deep understanding of lung cell wounding and repair, and discovery of relevant repair moieties for lung cells, should inspire the development of new therapies that are likely preventive and broadly effective for targeting injurious pulmonary diseases.
Keywords: acute respiratory distress syndrome; epithelium mesenchymal cross talk; lung cells; pulmonary fibrosis.
Copyright © 2017 the American Physiological Society.
Figures
Similar articles
-
Experimental models to study cell wounding and repair.Cell Physiol Biochem. 2010;25(1):71-80. doi: 10.1159/000272052. Epub 2009 Dec 22. Cell Physiol Biochem. 2010. PMID: 20054146 Review.
-
Mechanisms and consequences of oxidative stress in lung disease: therapeutic implications for an aging populace.Am J Physiol Lung Cell Mol Physiol. 2018 Apr 1;314(4):L642-L653. doi: 10.1152/ajplung.00275.2017. Epub 2017 Dec 14. Am J Physiol Lung Cell Mol Physiol. 2018. PMID: 29351446 Free PMC article. Review.
-
TRIM72 is required for effective repair of alveolar epithelial cell wounding.Am J Physiol Lung Cell Mol Physiol. 2014 Sep 15;307(6):L449-59. doi: 10.1152/ajplung.00172.2014. Epub 2014 Aug 8. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 25106429 Free PMC article.
-
New insights into the pathogenesis and treatment of idiopathic pulmonary fibrosis: a potential role for stem cells in the lung parenchyma and implications for therapy.Pharm Res. 2007 May;24(5):819-41. doi: 10.1007/s11095-006-9216-x. Epub 2007 Mar 1. Pharm Res. 2007. PMID: 17333393 Review.
-
Hypercapnia Alters Alveolar Epithelial Repair by a pH-Dependent and Adenylate Cyclase-Mediated Mechanism.Sci Rep. 2019 Jan 23;9(1):349. doi: 10.1038/s41598-018-36951-7. Sci Rep. 2019. PMID: 30674971 Free PMC article.
Cited by
-
Structural and signaling role of lipids in plasma membrane repair.Curr Top Membr. 2019;84:67-98. doi: 10.1016/bs.ctm.2019.07.001. Epub 2019 Jul 25. Curr Top Membr. 2019. PMID: 31610866 Free PMC article. Review.
-
The micromechanics of lung alveoli: structure and function of surfactant and tissue components.Histochem Cell Biol. 2018 Dec;150(6):661-676. doi: 10.1007/s00418-018-1747-9. Epub 2018 Nov 2. Histochem Cell Biol. 2018. PMID: 30390118 Free PMC article. Review.
-
Hidden Microatelectases Increase Vulnerability to Ventilation-Induced Lung Injury.Front Physiol. 2020 Sep 18;11:530485. doi: 10.3389/fphys.2020.530485. eCollection 2020. Front Physiol. 2020. PMID: 33071807 Free PMC article.
-
Alveolar Dynamics and Beyond - The Importance of Surfactant Protein C and Cholesterol in Lung Homeostasis and Fibrosis.Front Physiol. 2020 May 5;11:386. doi: 10.3389/fphys.2020.00386. eCollection 2020. Front Physiol. 2020. PMID: 32431623 Free PMC article. Review.
-
High-Throughput Microplate-Based Assay to Monitor Plasma Membrane Wounding and Repair.Front Cell Infect Microbiol. 2017 Jul 14;7:305. doi: 10.3389/fcimb.2017.00305. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28770170 Free PMC article.
References
-
- Akhmetshina A, Palumbo K, Dees C, Bergmann C, Venalis P, Zerr P, Horn A, Kireva T, Beyer C, Zwerina J, Schneider H, Sadowski A, Riener MO, MacDougald OA, Distler O, Schett G, Distler JH. Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nat Commun 3: 735, 2012. doi:10.1038/ncomms1734. - DOI - PMC - PubMed
-
- Albertine KH, Soulier MF, Wang Z, Ishizaka A, Hashimoto S, Zimmerman GA, Matthay MA, Ware LB. Fas and fas ligand are up-regulated in pulmonary edema fluid and lung tissue of patients with acute lung injury and the acute respiratory distress syndrome. Am J Pathol 161: 1783–1796, 2002. doi:10.1016/S0002-9440(10)64455-0. - DOI - PMC - PubMed
-
- Allewelt M, Coleman FT, Grout M, Priebe GP, Pier GB. Acquisition of expression of the Pseudomonas aeruginosa ExoU cytotoxin leads to increased bacterial virulence in a murine model of acute pneumonia and systemic spread. Infect Immun 68: 3998–4004, 2000. doi:10.1128/IAI.68.7.3998-4004.2000. - DOI - PMC - PubMed
-
- Anderson LV, Davison K, Moss JA, Young C, Cullen MJ, Walsh J, Johnson MA, Bashir R, Britton S, Keers S, Argov Z, Mahjneh I, Fougerousse F, Beckmann JS, Bushby KM. Dysferlin is a plasma membrane protein and is expressed early in human development. Hum Mol Genet 8: 855–861, 1999. doi:10.1093/hmg/8.5.855. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

