Modified Vaccinia Virus Ankara: History, Value in Basic Research, and Current Perspectives for Vaccine Development
- PMID: 28057259
- PMCID: PMC7112317
- DOI: 10.1016/bs.aivir.2016.07.001
Modified Vaccinia Virus Ankara: History, Value in Basic Research, and Current Perspectives for Vaccine Development
Abstract
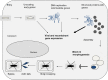
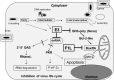
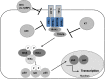
Safety tested Modified Vaccinia virus Ankara (MVA) is licensed as third-generation vaccine against smallpox and serves as a potent vector system for development of new candidate vaccines against infectious diseases and cancer. Historically, MVA was developed by serial tissue culture passage in primary chicken cells of vaccinia virus strain Ankara, and clinically used to avoid the undesirable side effects of conventional smallpox vaccination. Adapted to growth in avian cells MVA lost the ability to replicate in mammalian hosts and lacks many of the genes orthopoxviruses use to conquer their host (cell) environment. As a biologically well-characterized mutant virus, MVA facilitates fundamental research to elucidate the functions of poxvirus host-interaction factors. As extremely safe viral vectors MVA vaccines have been found immunogenic and protective in various preclinical infection models. Multiple recombinant MVA currently undergo clinical testing for vaccination against human immunodeficiency viruses, Mycobacterium tuberculosis or Plasmodium falciparum. The versatility of the MVA vector vaccine platform is readily demonstrated by the swift development of experimental vaccines for immunization against emerging infections such as the Middle East Respiratory Syndrome. Recent advances include promising results from the clinical testing of recombinant MVA-producing antigens of highly pathogenic avian influenza virus H5N1 or Ebola virus. This review summarizes our current knowledge about MVA as a unique strain of vaccinia virus, and discusses the prospects of exploiting this virus as research tool in poxvirus biology or as safe viral vector vaccine to challenge existing and future bottlenecks in vaccinology.
Keywords: Emergency vaccines; Infectious diseases; Poxvirus; Smallpox vaccine; Vaccine development; Viral vector vaccines; Virus–host interaction; Zoonoses.
© 2017 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Vaccinia vectors as candidate vaccines: the development of modified vaccinia virus Ankara for antigen delivery.Curr Drug Targets Infect Disord. 2003 Sep;3(3):263-71. doi: 10.2174/1568005033481123. Curr Drug Targets Infect Disord. 2003. PMID: 14529359 Review.
-
[Modified vaccinia virus ankara (MVA)--development as recombinant vaccine and prospects for use in veterinary medicine].Berl Munch Tierarztl Wochenschr. 2015 Nov-Dec;128(11-12):464-72. Berl Munch Tierarztl Wochenschr. 2015. PMID: 26697713 Review. German.
-
Expanding the repertoire of Modified Vaccinia Ankara-based vaccine vectors via genetic complementation strategies.PLoS One. 2009;4(5):e5445. doi: 10.1371/journal.pone.0005445. Epub 2009 May 6. PLoS One. 2009. PMID: 19421328 Free PMC article.
-
Protective efficacy of Modified Vaccinia virus Ankara in preclinical studies.Vaccine. 2013 Sep 6;31(39):4235-40. doi: 10.1016/j.vaccine.2013.03.016. Epub 2013 Mar 21. Vaccine. 2013. PMID: 23523402 Review.
-
Modified vaccinia virus Ankara as antigen delivery system: how can we best use its potential?Curr Opin Biotechnol. 2004 Dec;15(6):506-12. doi: 10.1016/j.copbio.2004.09.001. Curr Opin Biotechnol. 2004. PMID: 15560976 Free PMC article. Review.
Cited by
-
Cancer Vaccines for Triple-Negative Breast Cancer: A Systematic Review.Vaccines (Basel). 2023 Jan 9;11(1):146. doi: 10.3390/vaccines11010146. Vaccines (Basel). 2023. PMID: 36679991 Free PMC article. Review.
-
Knowledge domain and emerging trends in HIV-MTB co-infection from 2017 to 2022: A scientometric analysis based on VOSviewer and CiteSpace.Front Public Health. 2023 Feb 1;11:1044426. doi: 10.3389/fpubh.2023.1044426. eCollection 2023. Front Public Health. 2023. PMID: 36817921 Free PMC article. Review.
-
The Interaction of Human Papillomavirus Infection and Prostaglandin E2 Signaling in Carcinogenesis: A Focus on Cervical Cancer Therapeutics.Cells. 2022 Aug 15;11(16):2528. doi: 10.3390/cells11162528. Cells. 2022. PMID: 36010605 Free PMC article. Review.
-
Poxviral ANKR/F-box Proteins: Substrate Adapters for Ubiquitylation and More.Pathogens. 2022 Aug 3;11(8):875. doi: 10.3390/pathogens11080875. Pathogens. 2022. PMID: 36014996 Free PMC article. Review.
-
Distribution and absence of generalized lesions in mice following single dose intramuscular inoculation of the vaccine candidate MVA-MERS-S.Biologicals. 2018 Jul;54:58-62. doi: 10.1016/j.biologicals.2018.05.004. Biologicals. 2018. PMID: 29759890 Free PMC article.
References
-
- Alcami A., Smith G.L. A soluble receptor for interleukin-1β encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell. 1992;71:153. - PubMed
-
- Alcamí A., Smith G.L. Soluble interferon-γ receptors encoded by poxviruses. Comp. Immunol. Microbiol. Infect. Dis. 1996;19:305. - PubMed
-
- Alcamí A., Symons J.A., Collins P.D., Williams T.J., Smith G.L. Blockade of chemokine activity by a soluble chemokine binding protein from vaccinia virus. J. Immunol. 1998;160:624. - PubMed
-
- Anderson R.J., Hannan C.M., Gilbert S.C., Laidlaw S.M., Sheu E.G., Korten S., Sinden R., Butcher G.A., Skinner M.A., Hill A.V.S. Enhanced CD8 + T cell immune responses and protection elicited against plasmodium berghei malaria by prime boost immunization regimens using a novel attenuated fowlpox virus. J. Immunol. 2004;172:3094. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

