Common Variable Immunodeficiency patients with a phenotypic profile of immunosenescence present with thrombocytopenia
- PMID: 28054583
- PMCID: PMC5214528
- DOI: 10.1038/srep39710
Common Variable Immunodeficiency patients with a phenotypic profile of immunosenescence present with thrombocytopenia
Erratum in
-
Erratum: Common Variable Immunodeficiency patients with a phenotypic profile of immunosenescence present with thrombocytopenia.Sci Rep. 2017 Feb 27;7:42569. doi: 10.1038/srep42569. Sci Rep. 2017. PMID: 28240280 Free PMC article. No abstract available.
Abstract
Common variable immunodeficiency (CVID) is a heterogeneous group of diseases. Our aim was to define sub-groups of CVID patients with similar phenotypes and clinical characteristics. Using eight-color flow cytometry, we analyzed both B- and T-cell phenotypes in a cohort of 88 CVID patients and 48 healthy donors. A hierarchical clustering of probability binning "bins" yielded a separate cluster of 22 CVID patients with an abnormal phenotype. We showed coordinated proportional changes in naïve CD4+ T-cells (decreased), intermediate CD27- CD28+ CD4+ T-cells (increased) and CD21low B-cells (increased) that were stable for over three years. Moreover, the lymphocytes' immunophenotype in this patient cluster exhibited features of profound immunosenescence and chronic activation. Thrombocytopenia was only found in this cluster (36% of cases, manifested as Immune Thrombocytopenia (ITP) or Evans syndrome). Clinical complications more frequently found in these patients include lung fibrosis (in 59% of cases) and bronchiectasis (55%). The degree of severity of these symptoms corresponded to more deviation from normal levels with respect to CD21low B-cells, naïve CD4+ and CD27− CD28+ CD4+ T-cells. Next-generation sequencing did not reveal any common genetic background. We delineate a subgroup of CVID patients with activated and immunosenescent immunophenotype of lymphocytes and distinct set of clinical complications without common genetic background.
Figures




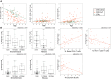
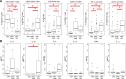
Similar articles
-
Profiling of polychromatic flow cytometry data on B-cells reveals patients' clusters in common variable immunodeficiency.Cytometry A. 2009 Nov;75(11):902-9. doi: 10.1002/cyto.a.20801. Cytometry A. 2009. PMID: 19802875
-
Age dependency and mutual relations in T and B lymphocyte abnormalities in common variable immunodeficiency patients.Clin Exp Immunol. 2006 Feb;143(2):373-9. doi: 10.1111/j.1365-2249.2006.02999.x. Clin Exp Immunol. 2006. PMID: 16412063 Free PMC article.
-
Low percentages of regulatory T cells in common variable immunodeficiency (CVID) patients with autoimmune diseases and its association with increased numbers of CD4+CD45RO+ T and CD21low B cells.Allergol Immunopathol (Madr). 2019 Sep-Oct;47(5):457-466. doi: 10.1016/j.aller.2019.01.003. Epub 2019 May 15. Allergol Immunopathol (Madr). 2019. PMID: 31103252
-
Autoimmunity and infection in common variable immunodeficiency (CVID).Autoimmun Rev. 2016 Sep;15(9):877-82. doi: 10.1016/j.autrev.2016.07.011. Epub 2016 Jul 6. Autoimmun Rev. 2016. PMID: 27392505 Review.
-
T-Cell Abnormalities in Common Variable Immunodeficiency.J Investig Allergol Clin Immunol. 2016;26(4):233-43. doi: 10.18176/jiaci.0069. Epub 2016 Apr 15. J Investig Allergol Clin Immunol. 2016. PMID: 27374799 Review.
Cited by
-
The clinical and immunological characteristics of common variable immunodeficiency in adults and older adults.North Clin Istanb. 2024 Jun 24;11(3):201-207. doi: 10.14744/nci.2023.49699. eCollection 2024. North Clin Istanb. 2024. PMID: 39005741 Free PMC article.
-
Predominantly Antibody-Deficient Patients With Non-infectious Complications Have Reduced Naive B, Treg, Th17, and Tfh17 Cells.Front Immunol. 2019 Nov 15;10:2593. doi: 10.3389/fimmu.2019.02593. eCollection 2019. Front Immunol. 2019. PMID: 31803177 Free PMC article.
-
Beyond monogenetic rare variants: tackling the low rate of genetic diagnoses in predominantly antibody deficiency.Cell Mol Immunol. 2021 Mar;18(3):588-603. doi: 10.1038/s41423-020-00520-8. Epub 2020 Aug 17. Cell Mol Immunol. 2021. PMID: 32801365 Free PMC article. Review.
-
B Cell Subsets in Colombian Adults with Predominantly Antibody Deficiencies, Bronchiectasis or Recurrent Pneumonia.Adv Respir Med. 2022 Jul 27;90(4):254-266. doi: 10.3390/arm90040035. Adv Respir Med. 2022. PMID: 36004955 Free PMC article.
-
The EuroFlow PID Orientation Tube for Flow Cytometric Diagnostic Screening of Primary Immunodeficiencies of the Lymphoid System.Front Immunol. 2019 Mar 4;10:246. doi: 10.3389/fimmu.2019.00246. eCollection 2019. Front Immunol. 2019. PMID: 30886612 Free PMC article.
References
-
- Gathmann B. et al.. Clinical picture and treatment of 2212 patients with common variable immunodeficiency. J. Allergy Clin. Immunol. 134, 116–126.e11 (2014). - PubMed
-
- Piqueras B. et al.. Common variable immunodeficiency patient classification based on impaired B cell memory differentiation correlates with clinical aspects. J. Clin. Immunol. 23, 385–400 (2003). - PubMed
-
- Warnatz K. et al.. Severe deficiency of switched memory B cells (CD27+IgM-IgD-) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood 99, 1544–1551 (2002). - PubMed
-
- Wehr C. et al.. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood 111, 77–85 (2008). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

