D-Amino Acids in the Nervous and Endocrine Systems
- PMID: 28053803
- PMCID: PMC5178360
- DOI: 10.1155/2016/6494621
D-Amino Acids in the Nervous and Endocrine Systems
Abstract
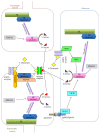
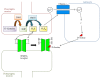
Amino acids are important components for peptides and proteins and act as signal transmitters. Only L-amino acids have been considered necessary in mammals, including humans. However, diverse D-amino acids, such as D-serine, D-aspartate, D-alanine, and D-cysteine, are found in mammals. Physiological roles of these D-amino acids not only in the nervous system but also in the endocrine system are being gradually revealed. N-Methyl-D-aspartate (NMDA) receptors are associated with learning and memory. D-Serine, D-aspartate, and D-alanine can all bind to NMDA receptors. H2S generated from D-cysteine reduces disulfide bonds in receptors and potentiates their activity. Aberrant receptor activity is related to diseases of the central nervous system (CNS), such as Alzheimer's disease, amyotrophic lateral sclerosis, and schizophrenia. Furthermore, D-amino acids are detected in parts of the endocrine system, such as the pineal gland, hypothalamus, pituitary gland, pancreas, adrenal gland, and testis. D-Aspartate is being investigated for the regulation of hormone release from various endocrine organs. Here we focused on recent findings regarding the synthesis and physiological functions of D-amino acids in the nervous and endocrine systems.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
D-Amino acids in mammalian endocrine tissues.Amino Acids. 2020 Sep;52(9):1263-1273. doi: 10.1007/s00726-020-02892-7. Epub 2020 Sep 15. Amino Acids. 2020. PMID: 32930873 Review.
-
Biosynthesis and Degradation of Free D-Amino Acids and Their Physiological Roles in the Periphery and Endocrine Glands.Biol Pharm Bull. 2024;47(3):562-579. doi: 10.1248/bpb.b23-00485. Biol Pharm Bull. 2024. PMID: 38432912 Review.
-
D-amino acids in the central nervous system in health and disease.Mol Genet Metab. 2005 Jul;85(3):168-80. doi: 10.1016/j.ymgme.2005.03.003. Mol Genet Metab. 2005. PMID: 15979028 Review.
-
Free d-Amino Acids in Salivary Gland in Rat.Biology (Basel). 2022 Mar 2;11(3):390. doi: 10.3390/biology11030390. Biology (Basel). 2022. PMID: 35336764 Free PMC article.
-
D-serine: the right or wrong isoform?Brain Res. 2011 Jul 15;1401:104-17. doi: 10.1016/j.brainres.2011.05.039. Epub 2011 May 23. Brain Res. 2011. PMID: 21676380 Review.
Cited by
-
Stereoselective Amine-omics Using Heavy Atom Isotope Labeled l- and d-Marfey's Reagents.J Am Soc Mass Spectrom. 2024 Jun 5;35(6):1217-1226. doi: 10.1021/jasms.4c00036. Epub 2024 Apr 29. J Am Soc Mass Spectrom. 2024. PMID: 38683793
-
Emerging nanosensor platforms and machine learning strategies toward rapid, point-of-need small-molecule metabolite detection and monitoring.Chem Sci. 2022 Sep 13;13(37):11009-11029. doi: 10.1039/d2sc02981b. eCollection 2022 Sep 28. Chem Sci. 2022. PMID: 36320477 Free PMC article. Review.
-
The Role of D-Serine and D-Aspartate in the Pathogenesis and Therapy of Treatment-Resistant Schizophrenia.Nutrients. 2022 Dec 2;14(23):5142. doi: 10.3390/nu14235142. Nutrients. 2022. PMID: 36501171 Free PMC article. Review.
-
New Insights Into the Mechanisms and Biological Roles of D-Amino Acids in Complex Eco-Systems.Front Microbiol. 2018 Apr 6;9:683. doi: 10.3389/fmicb.2018.00683. eCollection 2018. Front Microbiol. 2018. PMID: 29681896 Free PMC article. Review.
-
d-amino Acids in Health and Disease: A Focus on Cancer.Nutrients. 2019 Sep 12;11(9):2205. doi: 10.3390/nu11092205. Nutrients. 2019. PMID: 31547425 Free PMC article. Review.
References
-
- Meldrum B. S. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. Journal of Nutrition. 2000;130(4):1007S–1015S. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

