SNX-1 and RME-8 oppose the assembly of HGRS-1/ESCRT-0 degradative microdomains on endosomes
- PMID: 28053230
- PMCID: PMC5255583
- DOI: 10.1073/pnas.1612730114
SNX-1 and RME-8 oppose the assembly of HGRS-1/ESCRT-0 degradative microdomains on endosomes
Abstract
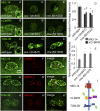
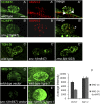
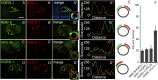
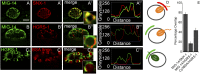
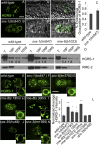
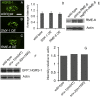
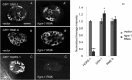
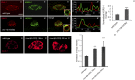
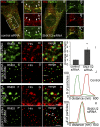
After endocytosis, transmembrane cargo reaches endosomes, where it encounters complexes dedicated to opposing functions: recycling and degradation. Microdomains containing endosomal sorting complexes required for transport (ESCRT)-0 component Hrs [hepatocyte growth factor-regulated tyrosine kinase substrate (HGRS-1) in Caenorhabditis elegans] mediate cargo degradation, concentrating ubiquitinated cargo and organizing the activities of ESCRT. At the same time, retromer associated sorting nexin one (SNX-1) and its binding partner, J-domain protein RME-8, sort cargo away from degradation, promoting cargo recycling to the Golgi. Thus, we hypothesized that there could be important regulatory interactions between retromer and ESCRT that balance degradative and recycling functions. Taking advantage of the naturally large endosomes of the C. elegans coelomocyte, we visualized complementary ESCRT-0 and RME-8/SNX-1 microdomains in vivo and assayed the ability of retromer and ESCRT microdomains to regulate one another. We found in snx-1(0) and rme-8(ts) mutants increased endosomal coverage and intensity of HGRS-1-labeled microdomains, as well as increased total levels of HGRS-1 bound to membranes. These effects are specific to SNX-1 and RME-8, as loss of other retromer components SNX-3 and vacuolar protein sorting-associated protein 35 (VPS-35) did not affect HGRS-1 microdomains. Additionally, knockdown of hgrs-1 had little to no effect on SNX-1 and RME-8 microdomains, suggesting directionality to the interaction. Separation of the functionally distinct ESCRT-0 and SNX-1/RME-8 microdomains was also compromised in the absence of RME-8 and SNX-1, a phenomenon we observed to be conserved, as depletion of Snx1 and Snx2 in HeLa cells also led to greater overlap of Rme-8 and Hrs on endosomes.
Keywords: Hrs; RME-8; SNX-1; clathrin; endosome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

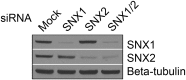
Similar articles
-
SNX-3 mediates retromer-independent tubular endosomal recycling by opposing EEA-1-facilitated trafficking.PLoS Genet. 2021 Jun 3;17(6):e1009607. doi: 10.1371/journal.pgen.1009607. eCollection 2021 Jun. PLoS Genet. 2021. PMID: 34081703 Free PMC article.
-
Regulation of endosomal clathrin and retromer-mediated endosome to Golgi retrograde transport by the J-domain protein RME-8.EMBO J. 2009 Nov 4;28(21):3290-302. doi: 10.1038/emboj.2009.272. Epub 2009 Sep 17. EMBO J. 2009. PMID: 19763082 Free PMC article.
-
Mutagenesis and structural modeling implicate RME-8 IWN domains as conformational control points.PLoS Genet. 2022 Oct 24;18(10):e1010296. doi: 10.1371/journal.pgen.1010296. eCollection 2022 Oct. PLoS Genet. 2022. PMID: 36279308 Free PMC article.
-
Endosomal microdomains: Formation and function.Curr Opin Cell Biol. 2020 Aug;65:86-95. doi: 10.1016/j.ceb.2020.02.018. Epub 2020 Apr 1. Curr Opin Cell Biol. 2020. PMID: 32247230 Free PMC article. Review.
-
Towards a molecular understanding of endosomal trafficking by Retromer and Retriever.Traffic. 2019 Jul;20(7):465-478. doi: 10.1111/tra.12649. Traffic. 2019. PMID: 30993794 Review.
Cited by
-
Commentary: BAG3 as a Mediator of Endosome Function and Tau Clearance.Neuroscience. 2023 May 10;518:4-9. doi: 10.1016/j.neuroscience.2022.05.002. Epub 2022 May 10. Neuroscience. 2023. PMID: 35550160 Free PMC article. Review.
-
Receptor-mediated endocytosis 8 (RME-8)/DNAJC13 is a novel positive modulator of autophagy and stabilizes cellular protein homeostasis.Cell Mol Life Sci. 2021 Jan;78(2):645-660. doi: 10.1007/s00018-020-03521-y. Epub 2020 Apr 22. Cell Mol Life Sci. 2021. PMID: 32322926 Free PMC article.
-
Sequence-dependent cargo recognition by SNX-BARs mediates retromer-independent transport of CI-MPR.J Cell Biol. 2017 Nov 6;216(11):3695-3712. doi: 10.1083/jcb.201703015. Epub 2017 Sep 21. J Cell Biol. 2017. PMID: 28935633 Free PMC article.
-
Transcytosis in the development and morphogenesis of epithelial tissues.EMBO J. 2021 May 3;40(9):e106163. doi: 10.15252/embj.2020106163. Epub 2021 Apr 1. EMBO J. 2021. PMID: 33792936 Free PMC article. Review.
-
Molecular interplays of the Entamoeba histolytica endosomal sorting complexes required for transport during phagocytosis.Front Cell Infect Microbiol. 2022 Oct 27;12:855797. doi: 10.3389/fcimb.2022.855797. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36389174 Free PMC article. Review.
References
-
- Mosesson Y, Mills GB, Yarden Y. Derailed endocytosis: An emerging feature of cancer. Nat Rev Cancer. 2008;8(11):835–850. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

