Tau Isoforms Imbalance Impairs the Axonal Transport of the Amyloid Precursor Protein in Human Neurons
- PMID: 28053030
- PMCID: PMC6705673
- DOI: 10.1523/JNEUROSCI.2305-16.2016
Tau Isoforms Imbalance Impairs the Axonal Transport of the Amyloid Precursor Protein in Human Neurons
Abstract
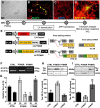
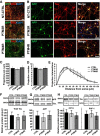
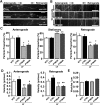
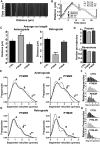
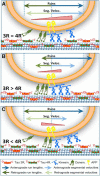
Tau, as a microtubule (MT)-associated protein, participates in key neuronal functions such as the regulation of MT dynamics, axonal transport, and neurite outgrowth. Alternative splicing of exon 10 in the tau primary transcript gives rise to protein isoforms with three (3R) or four (4R) MT binding repeats. Although tau isoforms are balanced in the normal adult human brain, imbalances in 3R:4R ratio have been tightly associated with the pathogenesis of several neurodegenerative disorders, yet the underlying molecular mechanisms remain elusive. Several studies exploiting tau overexpression and/or mutations suggested that perturbations in tau metabolism impair axonal transport. Nevertheless, no physiological model has yet demonstrated the consequences of altering the endogenous relative content of tau isoforms over axonal transport regulation. Here, we addressed this issue using a trans-splicing strategy that allows modulating tau exon 10 inclusion/exclusion in differentiated human-derived neurons. Upon changes in 3R:4R tau relative content, neurons showed no morphological changes, but live imaging studies revealed that the dynamics of the amyloid precursor protein (APP) were significantly impaired. Single trajectory analyses of the moving vesicles showed that predominance of 3R tau favored the anterograde movement of APP vesicles, increasing anterograde run lengths and reducing retrograde runs and segmental velocities. Conversely, the imbalance toward the 4R isoform promoted a retrograde bias by a significant reduction of anterograde velocities. These findings suggest that changes in 3R:4R tau ratio has an impact on the regulation of axonal transport and specifically in APP dynamics, which might link tau isoform imbalances with APP abnormal metabolism in neurodegenerative processes.
Significance statement: The tau protein has a relevant role in the transport of cargos throughout neurons. Dysfunction in tau metabolism underlies several neurological disorders leading to dementia. In the adult human brain, two tau isoforms are found in equal amounts, whereas changes in such equilibrium have been associated with neurodegenerative diseases. We investigated the role of tau in human neurons in culture and found that perturbations in the endogenous balance of tau isoforms were sufficient to impair the transport of the Alzheimer's disease-related amyloid precursor protein (APP), although neuronal morphology was normal. Our results provide evidence of a direct relationship between tau isoform imbalance and defects in axonal transport, which induce an abnormal APP metabolism with important implications in neurodegeneration.
Keywords: APP; Alzheimer's; axonal transport; splicing; tau; tauopathies.
Copyright © 2017 the authors 0270-6474/17/370059-12$15.00/0.
Figures
Comment in
-
Live-Cell Imaging Reveals Tau Isoforms Imbalance Disrupts Traffic of APP Vesicles in Human Neurons.J Neurosci. 2017 Feb 22;37(8):1968-1970. doi: 10.1523/JNEUROSCI.3688-16.2017. J Neurosci. 2017. PMID: 28228519 Free PMC article. No abstract available.
Similar articles
-
Modulation of Tau Isoforms Imbalance Precludes Tau Pathology and Cognitive Decline in a Mouse Model of Tauopathy.Cell Rep. 2018 Apr 17;23(3):709-715. doi: 10.1016/j.celrep.2018.03.079. Cell Rep. 2018. PMID: 29669277
-
Tau accumulation in the retina promotes early neuronal dysfunction and precedes brain pathology in a mouse model of Alzheimer's disease.Mol Neurodegener. 2017 Aug 3;12(1):58. doi: 10.1186/s13024-017-0199-3. Mol Neurodegener. 2017. PMID: 28774322 Free PMC article.
-
SMaRT modulation of tau isoforms rescues cognitive and motor impairments in a preclinical model of tauopathy.Front Bioeng Biotechnol. 2022 Oct 5;10:951384. doi: 10.3389/fbioe.2022.951384. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36277399 Free PMC article.
-
The Role of Tau Proteoforms in Health and Disease.Mol Neurobiol. 2023 Sep;60(9):5155-5166. doi: 10.1007/s12035-023-03387-8. Epub 2023 Jun 2. Mol Neurobiol. 2023. PMID: 37266762 Review.
-
Axonal transport, tau protein, and neurodegeneration in Alzheimer's disease.Neuromolecular Med. 2002;2(2):151-65. doi: 10.1385/NMM:2:2:151. Neuromolecular Med. 2002. PMID: 12428809 Review.
Cited by
-
Systemic Immune Dyshomeostasis Model and Pathways in Alzheimer's Disease.Front Aging Neurosci. 2019 Oct 23;11:290. doi: 10.3389/fnagi.2019.00290. eCollection 2019. Front Aging Neurosci. 2019. PMID: 31736740 Free PMC article.
-
Kinesin-1-mediated axonal transport of CB1 receptors is required for cannabinoid-dependent axonal growth and guidance.Development. 2020 Apr 20;147(8):dev184069. doi: 10.1242/dev.184069. Development. 2020. PMID: 32265198 Free PMC article.
-
Tau Isoforms: Gaining Insight into MAPT Alternative Splicing.Int J Mol Sci. 2022 Dec 6;23(23):15383. doi: 10.3390/ijms232315383. Int J Mol Sci. 2022. PMID: 36499709 Free PMC article. Review.
-
Delta-secretase (AEP) mediates tau-splicing imbalance and accelerates cognitive decline in tauopathies.J Exp Med. 2018 Dec 3;215(12):3038-3056. doi: 10.1084/jem.20180539. Epub 2018 Oct 29. J Exp Med. 2018. PMID: 30373880 Free PMC article.
-
High-resolution temporal and regional mapping of MAPT expression and splicing in human brain development.PLoS One. 2018 Apr 10;13(4):e0195771. doi: 10.1371/journal.pone.0195771. eCollection 2018. PLoS One. 2018. PMID: 29634760 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
