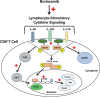
Bortezomib augments lymphocyte stimulatory cytokine signaling in the tumor microenvironment to sustain CD8+T cell antitumor function
- PMID: 28052005
- PMCID: PMC5352426
- DOI: 10.18632/oncotarget.14365
Bortezomib augments lymphocyte stimulatory cytokine signaling in the tumor microenvironment to sustain CD8+T cell antitumor function
Abstract
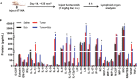
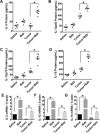
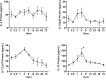
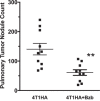
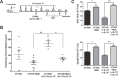
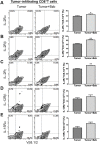
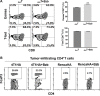
Tumor-induced immune tolerance poses a major challenge for therapeutic interventions aimed to manage cancer. We explored approaches to overcome T-cell suppression in murine breast and kidney adenocarcinomas, and lung fibrosarcoma expressing immunogenic antigens. We observed that treatment with a reversible proteasome inhibitor bortezomib (1 mg/kg body weight) in tumor-bearing mice significantly enhanced the expression of lymphocyte-stimulatory cytokines IL-2, IL-12, and IL-15. Notably, bortezomib administration reduced pulmonary nodules of mammary adenocarcinoma 4T1.2 expressing hemagglutinin (HA) model antigen (4T1HA) in mice. Neutralization of IL-12 and IL-15 cytokines with a regimen of blocking antibodies pre- and post-adoptive transfer of low-avidity HA518-526-specific CD8+T-cells following intravenous injection of 4T1HA cells increased the number of pulmonary tumor nodules. This neutralization effect was counteracted by the tumor metastasis-suppressing action of bortezomib treatments. In bortezomib-treated 4T1HA tumor-bearing mice, CD4+T-cells showed increased IL-2 production, CD11c+ dendritic cells showed increased IL-12 and IL-15 production, and HA-specific activated CD8+T-cells showed enhanced expression of IFNγ, granzyme-B and transcription factor eomesodermin. We also noted a trend of increased expression of IL-2, IL-12 and IL-15 receptors as well as increased phosphorylation of STAT5 in tumor-infiltrating CD8+T-cells following bortezomib treatment. Furthermore, bortezomib-treated CD8+T-cells showed increased phosphorylation of mitogen-activated protein kinase p38, and Akt, which was abrogated by phosphatidylinositide 3-kinase (PI3K) inhibitor. These data support the therapeutic potential of bortezomib in conjunction with other immunotherapies to augment the strength of convergent signals from CD8+T-cell signaling molecules including TCR, cytokine receptors and downstream PI3K/Akt/STAT5 pathways to sustain CD8+T-cell effector function in the tumor microenvironment.
Keywords: CD8+ T cells; adoptive cell therapy; cancer immunotherapy; immunosuppression; proteasome inhibition.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



Similar articles
-
Bortezomib enhances expression of effector molecules in anti-tumor CD8+ T lymphocytes by promoting Notch-nuclear factor-κB crosstalk.Oncotarget. 2015 Oct 20;6(32):32439-55. doi: 10.18632/oncotarget.5857. Oncotarget. 2015. PMID: 26431276 Free PMC article.
-
G-CSF and G-CSFR Modulate CD4 and CD8 T Cell Responses to Promote Colon Tumor Growth and Are Potential Therapeutic Targets.Front Immunol. 2020 Sep 15;11:1885. doi: 10.3389/fimmu.2020.01885. eCollection 2020. Front Immunol. 2020. PMID: 33042110 Free PMC article.
-
Breast cancer cell derived exosomes reduces glycolysis of activated CD8 + T cells in a AKT-mTOR dependent manner.Cell Biol Int. 2025 Jan;49(1):45-54. doi: 10.1002/cbin.12241. Epub 2024 Sep 16. Cell Biol Int. 2025. PMID: 39285531
-
Daratumumab and antineoplastic therapy versus antineoplastic therapy only for adults with newly diagnosed multiple myeloma ineligible for transplant.Cochrane Database Syst Rev. 2024 May 2;5(5):CD013595. doi: 10.1002/14651858.CD013595.pub2. Cochrane Database Syst Rev. 2024. PMID: 38695605 Review.
-
T cell landscape in the microenvironment of human solid tumors.Immunol Lett. 2024 Dec;270:106942. doi: 10.1016/j.imlet.2024.106942. Epub 2024 Oct 31. Immunol Lett. 2024. PMID: 39486594 Review.
Cited by
-
Ubiquitination in the regulation of inflammatory cell death and cancer.Cell Death Differ. 2021 Feb;28(2):591-605. doi: 10.1038/s41418-020-00708-5. Epub 2021 Jan 11. Cell Death Differ. 2021. PMID: 33432113 Free PMC article. Review.
-
Therapeutic Strategies against Epstein-Barr Virus-Associated Cancers Using Proteasome Inhibitors.Viruses. 2017 Nov 21;9(11):352. doi: 10.3390/v9110352. Viruses. 2017. PMID: 29160853 Free PMC article. Review.
-
The Proteasome Inhibitor Ixazomib Inhibits the Formation and Growth of Pulmonary and Abdominal Osteosarcoma Metastases in Mice.Cancers (Basel). 2020 May 11;12(5):1207. doi: 10.3390/cancers12051207. Cancers (Basel). 2020. PMID: 32403415 Free PMC article.
-
Bortezomib Sustains T Cell Function by Inducing miR-155-Mediated Downregulation of SOCS1 and SHIP1.Front Immunol. 2021 Feb 25;12:607044. doi: 10.3389/fimmu.2021.607044. eCollection 2021. Front Immunol. 2021. PMID: 33717088 Free PMC article.
-
The Impact of Induction Regimes on Immune Responses in Patients with Multiple Myeloma.Cancers (Basel). 2021 Aug 13;13(16):4090. doi: 10.3390/cancers13164090. Cancers (Basel). 2021. PMID: 34439244 Free PMC article. Review.
References
-
- Marcais A, Cherfils-Vicini J, Viant C, Degouve S, Viel S, Fenis A, Rabilloud J, Mayol K, Tavares A, Bienvenu J, Gangloff YG, Gilson E, Vivier E, Walzer T. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat Immunol. 2014;15:749–757. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- T32 HL007737/HL/NHLBI NIH HHS/United States
- U54 CA163069/CA/NCI NIH HHS/United States
- I01 BX002301/BX/BLRD VA/United States
- G12 MD007586/MD/NIMHD NIH HHS/United States
- U54 MD007586/MD/NIMHD NIH HHS/United States
- R24 DA036420/DA/NIDA NIH HHS/United States
- R01 CA175370/CA/NCI NIH HHS/United States
- R01 CA034590/CA/NCI NIH HHS/United States
- P50 CA090949/CA/NCI NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- U54 MD007593/MD/NIMHD NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
- SC1 CA182843/CA/NCI NIH HHS/United States
- IK6 BX005225/BX/BLRD VA/United States
- R01 CA116021/CA/NCI NIH HHS/United States
- R25 GM059994/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

