N-glycosylation converts non-glycoproteins into mannose receptor ligands and reveals antigen-specific T cell responses in vivo
- PMID: 28036287
- PMCID: PMC5351675
- DOI: 10.18632/oncotarget.14314
N-glycosylation converts non-glycoproteins into mannose receptor ligands and reveals antigen-specific T cell responses in vivo
Abstract
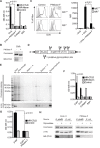
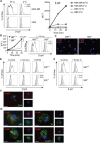
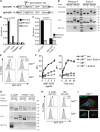
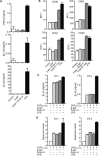
N-glycosylation is generally accepted to enhance the immunogenicity of antigens because of two main reasons. First, the attachment of glycans enables recognition by endocytic receptors like the mannose receptor (MR) and hence increased uptake by dendritic cells (DCs). Second, foreign glycans are postulated to be immunostimulatory and their recognition could induce DC activation. However, a direct comparison between the immunogenicity of N-glycosylated vs. de-glycosylated proteins in vivo and a direct effect of N-glycosylated antigens on the intrinsic capacity of DCs to activate T cells have not been assessed so far.To analyze whether enforced N-glycosylation is a suited strategy to enhance the immunogenicity of non-glycosylated antigens for vaccination studies, we targeted non-glycoproteins towards the MR by introduction of artificial N-glycosylation using the methylotrophic yeast Komagataella phaffii (previously termed Pichia pastoris). We could demonstrate that the introduction of a single N-X-S/T motif was sufficient for efficient MR-binding and internalization. However, addition of N-glycosylated proteins neither influenced DC maturation nor their general capacity to activate T cells, pointing out that enforced N-glycosylation does not increase the immunogenicity of the antigen per se. Additionally, increased antigen-specific cytotoxic T cell responses in vivo after injection of N-glycosylated compared to de-glycosylated proteins were observed but this effect strongly depended on the epitope tested. A beneficial effect of N-glycosylation on antibody production could not be detected, which might be due to MR-cross-linking on DCs and to concomitant differences in IL-6 production by CD4+ T cells.These observations point out that the effect of N-glycosylation on antigen immunogenicity can vary between different antigens and therefore might have important implications for the development of vaccines using K. phaffii.
Keywords: Komagataella phaffii; T cell activation; antigen presentation; cross-presentation; receptor-mediated endocytosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
The mannose receptor mediates uptake of soluble but not of cell-associated antigen for cross-presentation.J Immunol. 2006 Jun 1;176(11):6770-6. doi: 10.4049/jimmunol.176.11.6770. J Immunol. 2006. PMID: 16709836
-
Effect of O-linked glycosylation on the antigenicity, cellular uptake and trafficking in dendritic cells of recombinant Ber e 1.PLoS One. 2021 Apr 29;16(4):e0249876. doi: 10.1371/journal.pone.0249876. eCollection 2021. PLoS One. 2021. PMID: 33914740 Free PMC article.
-
Mannose receptor targeting of tumor antigen pmel17 to human dendritic cells directs anti-melanoma T cell responses via multiple HLA molecules.J Immunol. 2004 Mar 1;172(5):2845-52. doi: 10.4049/jimmunol.172.5.2845. J Immunol. 2004. PMID: 14978085
-
C-type lectins on dendritic cells: key modulators for the induction of immune responses.Biochem Soc Trans. 2008 Dec;36(Pt 6):1478-81. doi: 10.1042/BST0361478. Biochem Soc Trans. 2008. PMID: 19021579 Review.
-
Targeting the mannose receptor with mannosylated subunit vaccines.Curr Med Chem. 2014;21(30):3405-18. doi: 10.2174/0929867321666140826115552. Curr Med Chem. 2014. PMID: 25174924 Review.
Cited by
-
Mannose and Lactobionic Acid in Nasal Vaccination: Enhancing Antigen Delivery via C-Type Lectin Receptors.Pharmaceutics. 2024 Oct 8;16(10):1308. doi: 10.3390/pharmaceutics16101308. Pharmaceutics. 2024. PMID: 39458637 Free PMC article. Review.
-
The Mannose Receptor: From Endocytic Receptor and Biomarker to Regulator of (Meta)Inflammation.Front Immunol. 2021 Oct 14;12:765034. doi: 10.3389/fimmu.2021.765034. eCollection 2021. Front Immunol. 2021. PMID: 34721436 Free PMC article. Review.
-
Soluble mannose receptor induces proinflammatory macrophage activation and metaflammation.Proc Natl Acad Sci U S A. 2021 Aug 3;118(31):e2103304118. doi: 10.1073/pnas.2103304118. Proc Natl Acad Sci U S A. 2021. PMID: 34326259 Free PMC article.
-
Endoplasmic Reticulum-Associated Degradation-Dependent Processing in Cross-Presentation and Its Potential for Dendritic Cell Vaccinations: A Review.Pharmaceutics. 2020 Feb 13;12(2):153. doi: 10.3390/pharmaceutics12020153. Pharmaceutics. 2020. PMID: 32070016 Free PMC article. Review.
-
Distinct Subcellular Compartments of Dendritic Cells Used for Cross-Presentation.Int J Mol Sci. 2019 Nov 9;20(22):5606. doi: 10.3390/ijms20225606. Int J Mol Sci. 2019. PMID: 31717517 Free PMC article. Review.
References
-
- Burgdorf S, Kautz A, Böhnert V, Knolle PA, Kurts C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science. 2007;316:612–616. - PubMed
-
- Rauen J, Kreer C, Paillard A, van Duikeren S, Benckhuijsen WE, Camps MG, Valentijn A, Rob P M, Ossendorp F, Drijfhout JW, Arens R, Burgdorf S. Enhanced cross-presentation and improved CD8+ T cell responses after mannosylation of synthetic long peptides in mice. PLoS ONE. 2014;9:e103755. - PMC - PubMed
-
- Martinez-Pomares L. The mannose receptor. J Leukoc Biol. 2012;92:1177–1186. - PubMed
-
- Taylor ME, Bezouska K, Drickamer K. Contribution to ligand binding by multiple carbohydrate-recognition domains in the macrophage mannose receptor. J Biol Chem. 1992;267:1719–1726. - PubMed
-
- He L-Z, Crocker A, Lee J, Mendoza-Ramirez J, Wang X-T, Vitale LA, O'Neill T, Petromilli C, Zhang H-F, Lopez J, Rohrer D, Keler T, Clynes R. Antigenic targeting of the human mannose receptor induces tumor immunity. J Immunol. 2007;178:6259–6267. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

