Bispecific Antibodies as a Development Platform for New Concepts and Treatment Strategies
- PMID: 28036020
- PMCID: PMC5297683
- DOI: 10.3390/ijms18010048
Bispecific Antibodies as a Development Platform for New Concepts and Treatment Strategies
Abstract
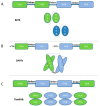
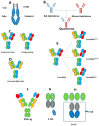
With the development of molecular cloning technology and the deep understanding of antibody engineering, there are diverse bispecific antibody formats from which to choose to pursue the optimal biological activity and clinical purpose. The single-chain-based bispecific antibodies usually bridge tumor cells with immune cells and form an immunological synapse because of their relatively small size. Bispecific antibodies in the IgG format include asymmetric bispecific antibodies and homodimerized bispecific antibodies, all of which have an extended blood half-life and their own crystalline fragment (Fc)-mediated functions. Besides retargeting effector cells to the site of cancer, new applications were established for bispecific antibodies. Bispecific antibodies that can simultaneously bind to cell surface antigens and payloads are a very ideal delivery system for therapeutic use. Bispecific antibodies that can inhibit two correlated signaling molecules at the same time can be developed to overcome inherent or acquired resistance and to be more efficient angiogenesis inhibitors. Bispecific antibodies can also be used to treat hemophilia A by mimicking the function of factor VIII. Bispecific antibodies also have broad application prospects in bone disorders and infections and diseases of the central nervous system. The latest developments of the formats and application of bispecific antibodies will be reviewed. Furthermore, the challenges and perspectives are summarized in this review.
Keywords: angiogenesis inhibitors; bispecific T cell engagers (BiTEs); bispecific antibodies; delivery system; drug resistance; immunological synapse; immunotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Bispecific antibodies for cancer immunotherapy: Current perspectives.BioDrugs. 2010 Apr 1;24(2):89-98. doi: 10.2165/11530960-000000000-00000. BioDrugs. 2010. PMID: 20199124 Review.
-
Rearranging the domain order of a diabody-based IgG-like bispecific antibody enhances its antitumor activity and improves its degradation resistance and pharmacokinetics.MAbs. 2014;6(5):1243-54. doi: 10.4161/mabs.29445. Epub 2014 Oct 30. MAbs. 2014. PMID: 25517309 Free PMC article.
-
Alternative molecular formats and therapeutic applications for bispecific antibodies.Mol Immunol. 2015 Oct;67(2 Pt A):95-106. doi: 10.1016/j.molimm.2015.01.003. Epub 2015 Jan 27. Mol Immunol. 2015. PMID: 25637431 Review.
-
Bispecific antibodies for cancer therapy.Immunotherapy. 2009 Mar;1(2):211-22. doi: 10.2217/1750743X.1.2.211. Immunotherapy. 2009. PMID: 20635943 Review.
-
Recombinant bispecific antibodies for cancer therapy.Acta Pharmacol Sin. 2005 Jan;26(1):1-9. doi: 10.1111/j.1745-7254.2005.00008.x. Acta Pharmacol Sin. 2005. PMID: 15659107 Review.
Cited by
-
CAR-T cell therapy: a potential new strategy against prostate cancer.J Immunother Cancer. 2019 Oct 16;7(1):258. doi: 10.1186/s40425-019-0741-7. J Immunother Cancer. 2019. PMID: 31619289 Free PMC article. Review.
-
Recent Advances in the Development of Monoclonal Antibodies and Next-Generation Antibodies.Immunohorizons. 2023 Dec 1;7(12):886-897. doi: 10.4049/immunohorizons.2300102. Immunohorizons. 2023. PMID: 38149884 Free PMC article.
-
Molecular pathogenesis of a novel Met394Thr variant causing hemophilia B.Mol Genet Genomic Med. 2023 May;11(5):e2147. doi: 10.1002/mgg3.2147. Epub 2023 Feb 16. Mol Genet Genomic Med. 2023. PMID: 36795372 Free PMC article.
-
Bispecific Antibodies in Cancer Immunotherapy: A Novel Response to an Old Question.Pharmaceutics. 2022 Jun 11;14(6):1243. doi: 10.3390/pharmaceutics14061243. Pharmaceutics. 2022. PMID: 35745815 Free PMC article. Review.
-
Development of a Bispecific Nanobody Targeting CD20 on B-Cell Lymphoma Cells and CD3 on T Cells.Vaccines (Basel). 2022 Aug 17;10(8):1335. doi: 10.3390/vaccines10081335. Vaccines (Basel). 2022. PMID: 36016223 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

