A Quininib Analogue and Cysteinyl Leukotriene Receptor Antagonist Inhibits Vascular Endothelial Growth Factor (VEGF)-independent Angiogenesis and Exerts an Additive Antiangiogenic Response with Bevacizumab
- PMID: 28035003
- PMCID: PMC5339742
- DOI: 10.1074/jbc.M116.747766
A Quininib Analogue and Cysteinyl Leukotriene Receptor Antagonist Inhibits Vascular Endothelial Growth Factor (VEGF)-independent Angiogenesis and Exerts an Additive Antiangiogenic Response with Bevacizumab
Abstract
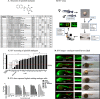
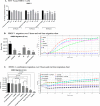
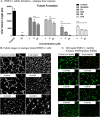
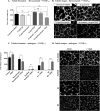
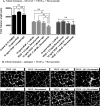
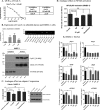
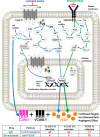
Excess blood vessel growth contributes to the pathology of metastatic cancers and age-related retinopathies. Despite development of improved treatments, these conditions are associated with high economic costs and drug resistance. Bevacizumab (Avastin®), a monoclonal antibody against vascular endothelial growth factor (VEGF), is used clinically to treat certain types of metastatic cancers. Unfortunately, many patients do not respond or inevitably become resistant to bevacizumab, highlighting the need for more effective antiangiogenic drugs with novel mechanisms of action. Previous studies discovered quininib, an antiangiogenic small molecule antagonist of cysteinyl leukotriene receptors 1 and 2 (CysLT1 and CysLT2). Here, we screened a series of quininib analogues and identified a more potent antiangiogenic novel chemical entity (IUPAC name (E)-2-(2-quinolin-2-yl-vinyl)-benzene-1,4-diol HCl) hereafter designated Q8. Q8 inhibits developmental angiogenesis in Tg(fli1:EGFP) zebrafish and inhibits human microvascular endothelial cell (HMEC-1) proliferation, tubule formation, and migration. Q8 elicits antiangiogenic effects in a VEGF-independent in vitro model of angiogenesis and exerts an additive antiangiogenic response with the anti-VEGF biologic bevacizumab. Cell-based receptor binding assays confirm that Q8 is a CysLT1 antagonist and is sufficient to reduce cellular levels of NF-κB and calpain-2 and secreted levels of the proangiogenic proteins intercellular adhesion molecule-1, vascular cell adhesion protein-1, and VEGF. Distinct reductions of VEGF by bevacizumab explain the additive antiangiogenic effects observed in combination with Q8. In summary, Q8 is a more effective antiangiogenic drug compared with quininib. The VEGF-independent activity coupled with the additive antiangiogenic response observed in combination with bevacizumab demonstrates that Q8 offers an alternative therapeutic strategy to combat resistance associated with conventional anti-VEGF therapies.
Keywords: G protein-coupled receptor (GPCR); angiogenesis; calpain; cell migration; colorectal cancer; drug development; endothelial cell; leukotriene; pharmacology; vascular endothelial growth factor (VEGF).
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
J. O. and B. N. K. are inventors on United States Patent 8916586 B2, and A. L. R., J. O., and B. N. K. are inventors on United States Patent 9388138 B2
Figures
Similar articles
-
Phenotype-based Discovery of 2-[(E)-2-(Quinolin-2-yl)vinyl]phenol as a Novel Regulator of Ocular Angiogenesis.J Biol Chem. 2016 Apr 1;291(14):7242-55. doi: 10.1074/jbc.M115.710665. Epub 2016 Feb 4. J Biol Chem. 2016. PMID: 26846851 Free PMC article.
-
Preclinical validation of the small molecule drug quininib as a novel therapeutic for colorectal cancer.Sci Rep. 2016 Oct 14;6:34523. doi: 10.1038/srep34523. Sci Rep. 2016. PMID: 27739445 Free PMC article.
-
Discovery and Development of the Quininib Series of Ocular Drugs.J Ocul Pharmacol Ther. 2022 Jan-Feb;38(1):33-42. doi: 10.1089/jop.2021.0074. J Ocul Pharmacol Ther. 2022. PMID: 35089801
-
Antiangiogenic therapy in malignant glioma: promise and challenge.Curr Pharm Des. 2007;13(35):3545-58. doi: 10.2174/138161207782794130. Curr Pharm Des. 2007. PMID: 18220791 Review.
-
Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications.Semin Oncol. 2002 Dec;29(6 Suppl 16):10-4. doi: 10.1053/sonc.2002.37264. Semin Oncol. 2002. PMID: 12516033 Review.
Cited by
-
Identification of mundoserone by zebrafish in vivo screening as a natural product with anti-angiogenic activity.Exp Ther Med. 2018 Dec;16(6):4562-4568. doi: 10.3892/etm.2018.6748. Epub 2018 Sep 17. Exp Ther Med. 2018. PMID: 30542405 Free PMC article.
-
Zileuton, a 5-Lipoxygenase Inhibitor, Exerts Anti-Angiogenic Effect by Inducing Apoptosis of HUVEC via BK Channel Activation.Cells. 2019 Sep 30;8(10):1182. doi: 10.3390/cells8101182. Cells. 2019. PMID: 31575085 Free PMC article.
-
Orthogonal Drug Pooling Enhances Phenotype-Based Discovery of Ocular Antiangiogenic Drugs in Zebrafish Larvae.Front Pharmacol. 2019 May 24;10:508. doi: 10.3389/fphar.2019.00508. eCollection 2019. Front Pharmacol. 2019. PMID: 31178719 Free PMC article. Review.
-
High Cysteinyl Leukotriene Receptor 1 Expression Correlates with Poor Survival of Uveal Melanoma Patients and Cognate Antagonist Drugs Modulate the Growth, Cancer Secretome, and Metabolism of Uveal Melanoma Cells.Cancers (Basel). 2020 Oct 13;12(10):2950. doi: 10.3390/cancers12102950. Cancers (Basel). 2020. PMID: 33066024 Free PMC article.
-
1,4-dihydroxy quininib modulates the secretome of uveal melanoma tumour explants and a marker of oxidative phosphorylation in a metastatic xenograft model.Front Med (Lausanne). 2023 Jan 9;9:1036322. doi: 10.3389/fmed.2022.1036322. eCollection 2022. Front Med (Lausanne). 2023. PMID: 36698840 Free PMC article.
References
-
- Otrock Z. K., Mahfouz R. A., Makarem J. A., and Shamseddine A. I. (2007) Understanding the biology of angiogenesis: review of the most important molecular mechanisms. Blood Cells Mol. Dis. 39, 212–220 - PubMed
-
- Carmeliet P. (2003) Angiogenesis in health and disease. Nat. Med. 9, 653–660 - PubMed
-
- Carmeliet P. (2005) Angiogenesis in life, disease and medicine. Nature 438, 932–936 - PubMed
-
- World Health Organization (2012) GLOBOCAN 2012—Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012, International Agency for Research on Cancer, Lyon, France
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

