Distinct complexes of yeast Snx4 family SNX-BARs mediate retrograde trafficking of Snc1 and Atg27
- PMID: 28026081
- PMCID: PMC5262529
- DOI: 10.1111/tra.12462
Distinct complexes of yeast Snx4 family SNX-BARs mediate retrograde trafficking of Snc1 and Atg27
Abstract
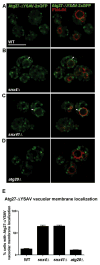
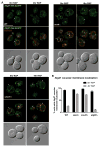
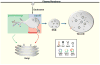
The yeast SNX4 sub-family of sorting nexin containing a Bin-Amphiphysin-Rvs domain (SNX-BAR) proteins, Snx4/Atg24, Snx41 and Atg20/Snx42, are required for endocytic recycling and selective autophagy. Here, we show that Snx4 forms 2 functionally distinct heterodimers: Snx4-Atg20 and Snx4-Snx41. Each heterodimer coats an endosome-derived tubule that mediates retrograde sorting of distinct cargo; the v-SNARE, Snc1, is a cargo of the Snx4-Atg20 pathway, and Snx4-Snx41 mediates retrograde sorting of Atg27, an integral membrane protein implicated in selective autophagy. Live cell imaging of individual endosomes shows that Snx4 and the Vps5-Vps17 retromer SNX-BAR heterodimer operate concurrently on a maturing endosome. Consistent with this, the yeast dynamin family protein, Vps1, which was previously shown to promote fission of retromer-coated tubules, promotes fission of Snx4-Atg20 coated tubules. The results indicate that the yeast SNX-BAR proteins coat 3 distinct types of endosome-derived carriers that mediate endosome-to-Golgi retrograde trafficking.
Keywords: SNX-BAR; Atg27; Vps1; autophagy; endosome; retromer.
© 2016 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures

Similar articles
-
Lipid trafficking by yeast Snx4 family SNX-BAR proteins promotes autophagy and vacuole membrane fusion.Mol Biol Cell. 2018 Sep 1;29(18):2190-2200. doi: 10.1091/mbc.E17-12-0743. Epub 2018 Jun 27. Mol Biol Cell. 2018. PMID: 29949447 Free PMC article.
-
SNX-BAR-mediated endosome tubulation is co-ordinated with endosome maturation.Traffic. 2012 Jan;13(1):94-107. doi: 10.1111/j.1600-0854.2011.01297.x. Epub 2011 Oct 31. Traffic. 2012. PMID: 21973056
-
Fission of SNX-BAR-coated endosomal retrograde transport carriers is promoted by the dynamin-related protein Vps1.J Cell Biol. 2014 Mar 3;204(5):793-806. doi: 10.1083/jcb.201309084. Epub 2014 Feb 24. J Cell Biol. 2014. PMID: 24567361 Free PMC article.
-
Updated Insight into the Physiological and Pathological Roles of the Retromer Complex.Int J Mol Sci. 2017 Jul 25;18(8):1601. doi: 10.3390/ijms18081601. Int J Mol Sci. 2017. PMID: 28757549 Free PMC article. Review.
-
Retromer and the cation-independent mannose 6-phosphate receptor-Time for a trial separation?Traffic. 2018 Feb;19(2):150-152. doi: 10.1111/tra.12542. Epub 2017 Dec 21. Traffic. 2018. PMID: 29135085 Free PMC article. Review.
Cited by
-
Lifespan Increase of Podospora anserina by Oleic Acid Is Linked to Alterations in Energy Metabolism, Membrane Trafficking and Autophagy.Cells. 2022 Feb 2;11(3):519. doi: 10.3390/cells11030519. Cells. 2022. PMID: 35159328 Free PMC article.
-
Lipid trafficking by yeast Snx4 family SNX-BAR proteins promotes autophagy and vacuole membrane fusion.Mol Biol Cell. 2018 Sep 1;29(18):2190-2200. doi: 10.1091/mbc.E17-12-0743. Epub 2018 Jun 27. Mol Biol Cell. 2018. PMID: 29949447 Free PMC article.
-
Autophagy as an on-ramp to scientific discovery.Autophagy. 2021 Apr;17(4):837-839. doi: 10.1080/15548627.2020.1769972. Epub 2020 Jun 16. Autophagy. 2021. PMID: 32543335 Free PMC article.
-
Structure and function of yeast Atg20, a sorting nexin that facilitates autophagy induction.Proc Natl Acad Sci U S A. 2017 Nov 21;114(47):E10112-E10121. doi: 10.1073/pnas.1708367114. Epub 2017 Nov 7. Proc Natl Acad Sci U S A. 2017. PMID: 29114050 Free PMC article.
-
Atg9 proteins, not so different after all.Autophagy. 2018;14(8):1456-1459. doi: 10.1080/15548627.2018.1477382. Epub 2018 Jul 23. Autophagy. 2018. PMID: 29966469 Free PMC article.
References
-
- Maxfield FR, McGraw TE. Endocytic recycling. Nature reviews Molecular cell biology. 2004;5(2):121–132. - PubMed
-
- van Weering JR, Cullen PJ. Membrane-associated cargo recycling by tubule-based endosomal sorting. Seminars in cell & developmental biology. 2014;31:40–47. - PubMed
-
- Peter BJ, Kent HM, Mills IG, et al. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303(5657):495–499. - PubMed
-
- McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438(7068):590–596. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

