Inherent variability of cancer-specific aneuploidy generates metastases
- PMID: 28018487
- PMCID: PMC5160004
- DOI: 10.1186/s13039-016-0297-x
Inherent variability of cancer-specific aneuploidy generates metastases
Abstract
Background: The genetic basis of metastasis is still unclear because metastases carry individual karyotypes and phenotypes, rather than consistent mutations, and are rare compared to conventional mutation. There is however correlative evidence that metastasis depends on cancer-specific aneuploidy, and that metastases are karyotypically related to parental cancers. Accordingly we propose that metastasis is a speciation event. This theory holds that cancer-specific aneuploidy varies the clonal karyotypes of cancers automatically by unbalancing thousands of genes, and that rare variants form new autonomous subspecies with metastatic or other non-parental phenotypes like drug-resistance - similar to conventional subspeciation.
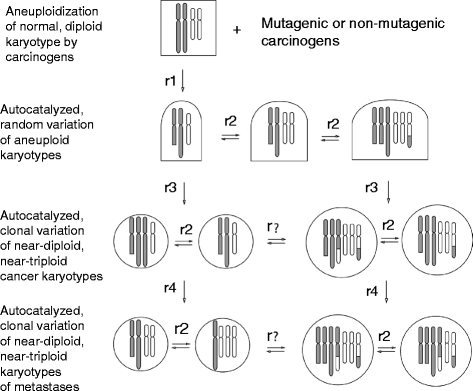
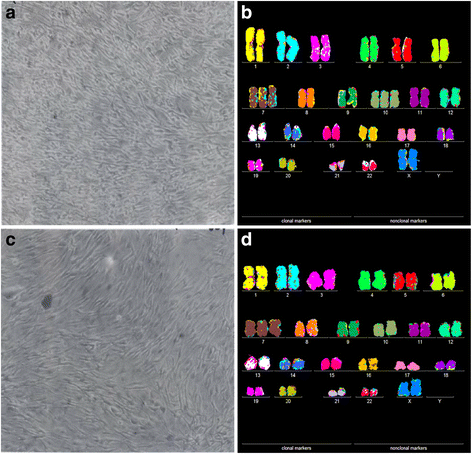
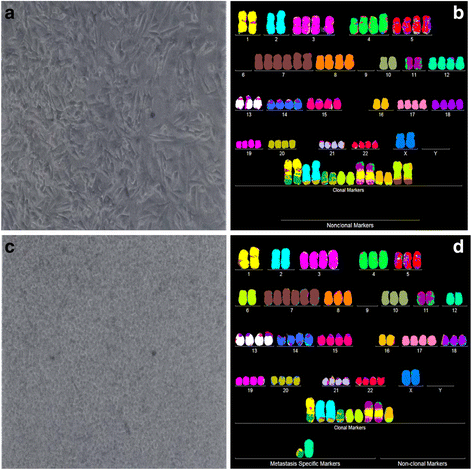
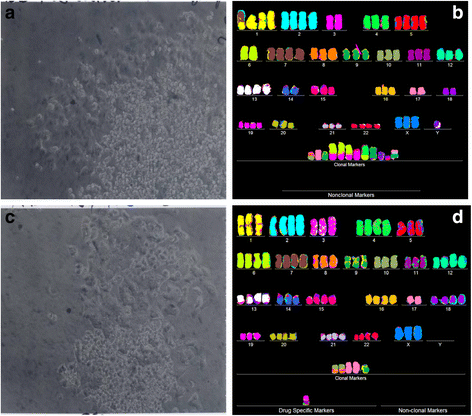
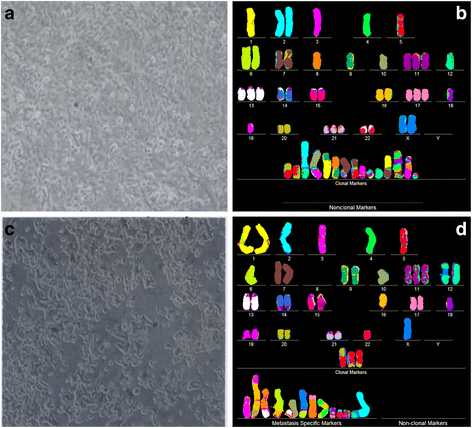
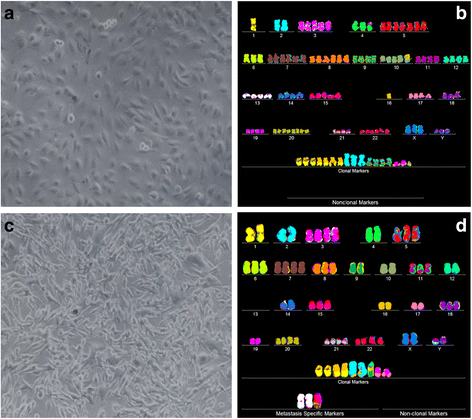
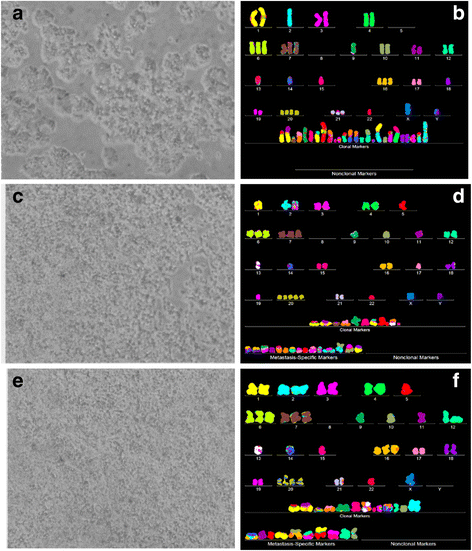
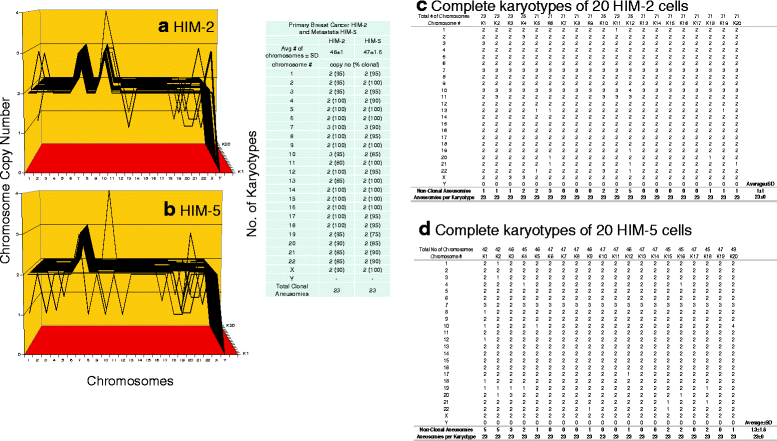
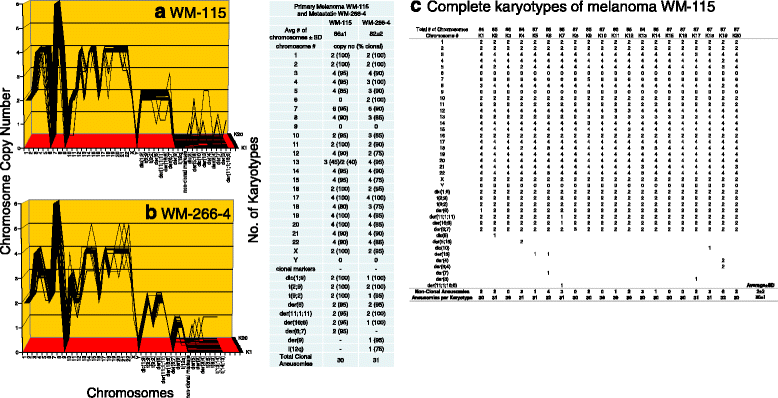
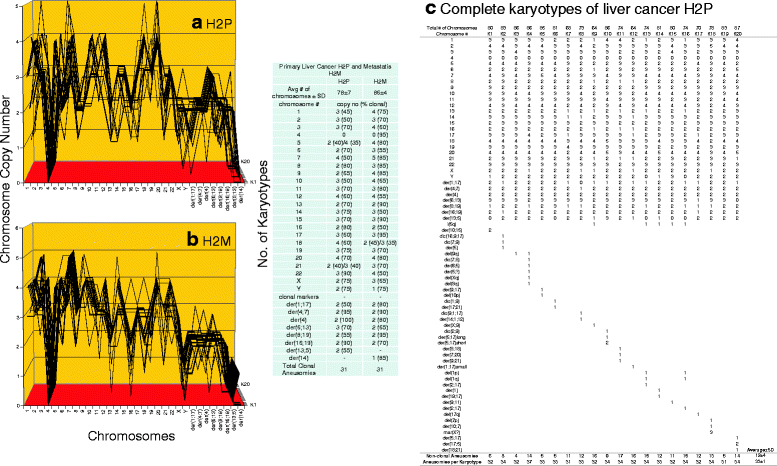
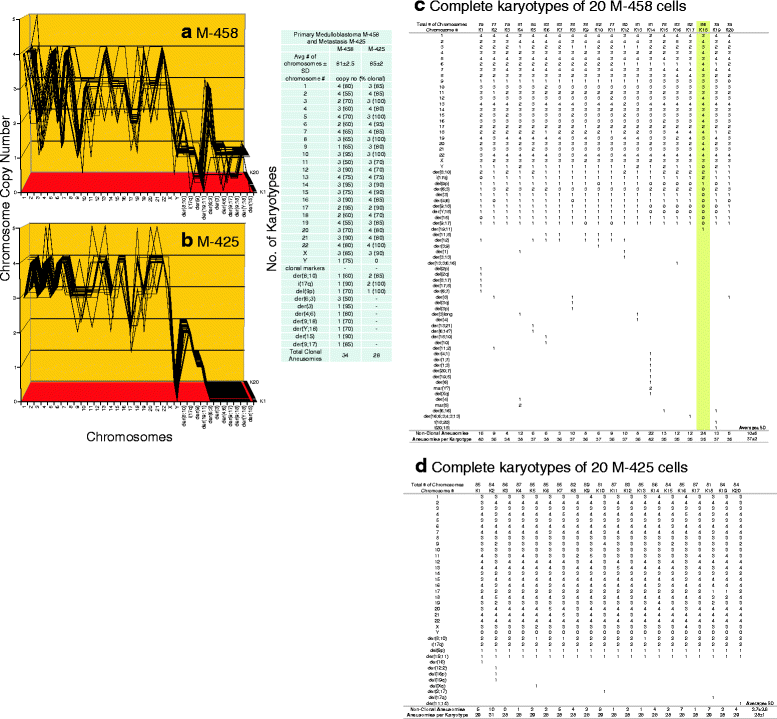
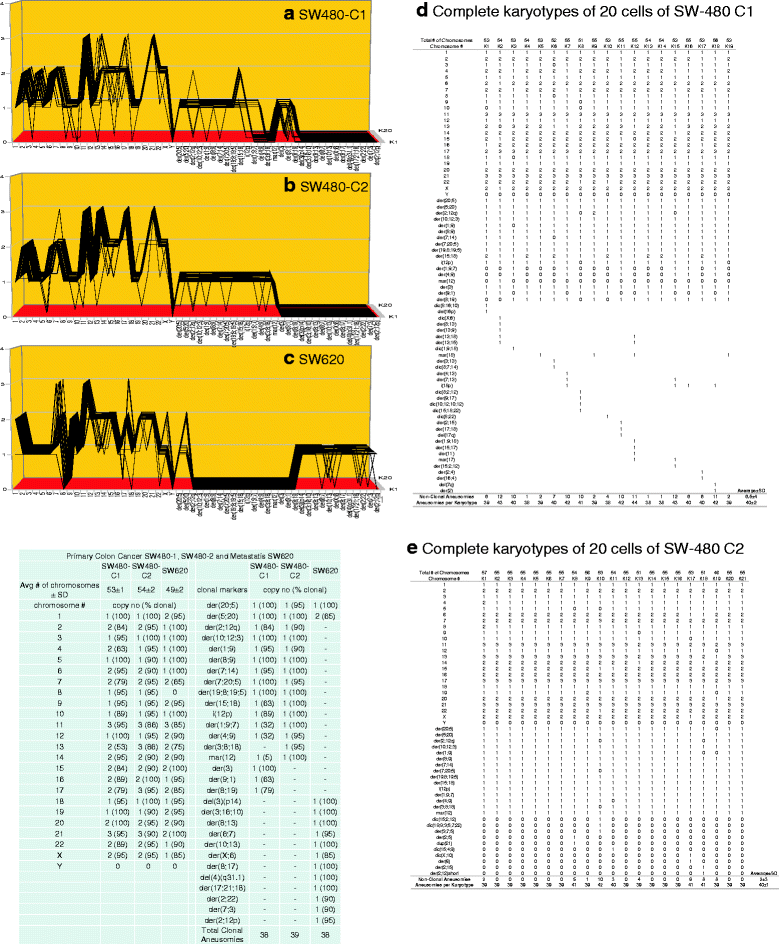
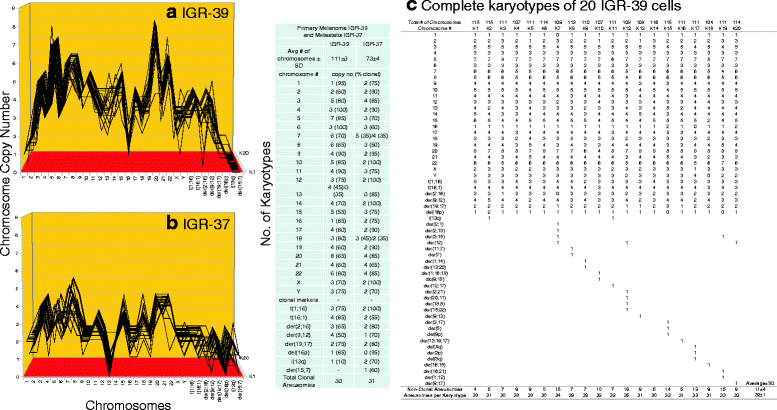
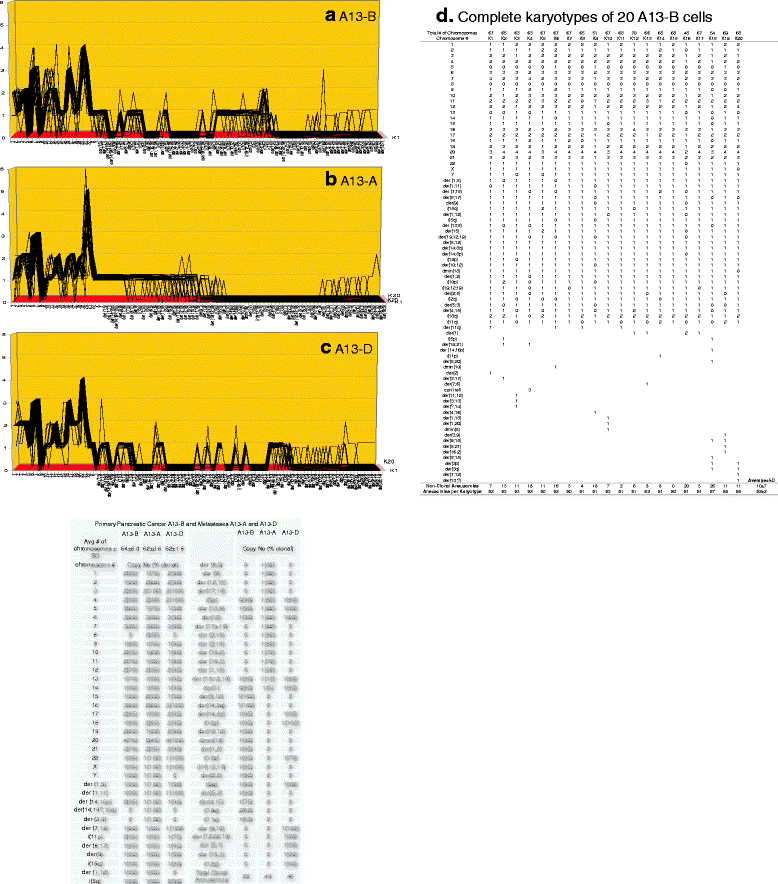
Results: To test this theory, we analyzed the karyotypic and morphological relationships between seven cancers and corresponding metastases. We found (1) that the cellular phenotypes of metastases were closely related to those of parental cancers, (2) that metastases shared 29 to 96% of their clonal karyotypic elements or aneusomies with the clonal karyotypes of parental cancers and (3) that, unexpectedly, the karyotypic complexity of metastases was very similar to that of the parental cancer. This suggests that metastases derive cancer-specific autonomy by conserving the overall complexity of the parental karyotype. We deduced from these results that cancers cause metastases by karyotypic variations and selection for rare metastatic subspecies. Further we asked whether metastases with multiple metastasis-specific aneusomies are assembled in one or multiple, sequential steps. Since (1) no stable karyotypic intermediates of metastases were observed in cancers here and previously by others, and (2) the karyotypic complexities of cancers are conserved in metastases, we concluded that metastases are generated from cancers in one step - like subspecies in conventional speciation.
Conclusions: We conclude that the risk of cancers to metastasize is proportional to the degree of cancer-specific aneuploidy, because aneuploidy catalyzes the generation of subspecies, including metastases, at aneuploidy-dependent rates. Since speciation by random chromosomal rearrangements and selection is unpredictable, the theory that metastases are karyotypic subspecies of cancers also explains Foulds' rules, which hold that the origins of metastases are "abrupt" and that their phenotypes are "unpredictable."
Figures

Similar articles
-
Speciation Theory of Carcinogenesis Explains Karyotypic Individuality and Long Latencies of Cancers.Genes (Basel). 2018 Aug 9;9(8):402. doi: 10.3390/genes9080402. Genes (Basel). 2018. PMID: 30096943 Free PMC article.
-
Karyotypic evolutions of cancer species in rats during the long latent periods after injection of nitrosourea.Mol Cytogenet. 2014 Dec 16;7(1):71. doi: 10.1186/s13039-014-0071-x. eCollection 2014. Mol Cytogenet. 2014. PMID: 25614763 Free PMC article.
-
Origin of metastases: subspecies of cancers generated by intrinsic karyotypic variations.Cell Cycle. 2012 Mar 15;11(6):1151-66. doi: 10.4161/cc.11.6.19580. Epub 2012 Mar 15. Cell Cycle. 2012. PMID: 22377695
-
The chromosomal basis of cancer.Cell Oncol. 2005;27(5-6):293-318. doi: 10.1155/2005/951598. Cell Oncol. 2005. PMID: 16373963 Free PMC article. Review.
-
Aneuploidy, the primary cause of the multilateral genomic instability of neoplastic and preneoplastic cells.IUBMB Life. 2004 Feb;56(2):65-81. doi: 10.1080/15216540410001667902. IUBMB Life. 2004. PMID: 15085930 Review.
Cited by
-
What Is Karyotype Coding and Why Is Genomic Topology Important for Cancer and Evolution?Front Genet. 2019 Nov 1;10:1082. doi: 10.3389/fgene.2019.01082. eCollection 2019. Front Genet. 2019. PMID: 31737054 Free PMC article. Review.
-
Genome Chaos, Information Creation, and Cancer Emergence: Searching for New Frameworks on the 50th Anniversary of the "War on Cancer".Genes (Basel). 2021 Dec 31;13(1):101. doi: 10.3390/genes13010101. Genes (Basel). 2021. PMID: 35052441 Free PMC article. Review.
-
Speciation Theory of Carcinogenesis Explains Karyotypic Individuality and Long Latencies of Cancers.Genes (Basel). 2018 Aug 9;9(8):402. doi: 10.3390/genes9080402. Genes (Basel). 2018. PMID: 30096943 Free PMC article.
-
The potential role of leptin in tumor invasion and metastasis.Cytokine Growth Factor Rev. 2017 Dec;38:80-97. doi: 10.1016/j.cytogfr.2017.11.002. Epub 2017 Nov 11. Cytokine Growth Factor Rev. 2017. PMID: 29158066 Free PMC article. Review.
-
Correlation between polymorphism of TYMS gene and toxicity response to treatment with 5-fluoruracil and capecitabine.Eur J Transl Myol. 2020 Aug 4;30(3):8970. doi: 10.4081/ejtm.2020.8970. eCollection 2020 Sep 30. Eur J Transl Myol. 2020. PMID: 33117504 Free PMC article.
References
-
- New Oxford American Dictionary: 2010, 2013 by Oxford University Press, Inc
-
- Foulds L. Multiple etiologic factors in neoplastic development. Cancer Res. 1965;25(8):1339–1347. - PubMed
-
- Weinberg RA. The biology of cancer, Second edition. New York; London: Garland Science; 2014.
-
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. New York: Garland; 2014.
-
- Foulds L. Tumor progression: a review. Cancer Res. 1954;14:327–339. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

