Obesity: An Immunometabolic Perspective
- PMID: 28018292
- PMCID: PMC5149556
- DOI: 10.3389/fendo.2016.00157
Obesity: An Immunometabolic Perspective
Abstract
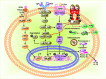
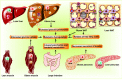
Obesity, characterized by chronic activation of inflammatory pathways, is a critical factor contributing to insulin resistance (IR) and type 2 diabetes (T2D). Free fatty acids (FFAs) are increased in obesity and are implicated as proximate causes of IR and induction of inflammatory signaling in adipose, liver, muscle, and pancreas. Cells of the innate immune system produce cytokines, and other factors that affect insulin signaling and result in the development of IR. In the lean state, adipose tissue is populated by adipose tissue macrophage of the anti-inflammatory M2 type (ATM2) and natural killer (NK) cells; this maintains the insulin-sensitive phenotype because ATM2 cells secrete IL10. In contrast, obesity induces lipolysis and release of pro-inflammatory FFAs and factors, such as chemokine (C-C motif) ligand 2 (CCL2) and tumor necrosis factor alpha (TNF-α), which recruit blood monocytes in adipose tissue, where they are converted to macrophages of the highly pro-inflammatory M1-type (ATM1). Activated ATM1 produce large amounts of pro-inflammatory mediators such as TNF-α, interleukin-1β, IL-6, leukotriene B4, nitric oxide (NO), and resistin that work in a paracrine fashion and cause IR in adipose tissue. In the liver, both pro-inflammatory Kupffer cells (M1-KCs) and recruited hepatic macrophages (Ly6Chigh) contribute to decreased hepatic insulin sensitivity. The present mini-review will update the bidirectional interaction between the immune system and obesity-induced changes in metabolism in adipose tissue and liver and the metabolic consequences thereof.
Keywords: ER stress; insulin resistance; macrophages; non-alcoholic fatty liver diseases; obesity; reactive oxygen species; type 2 diabetes.
Figures
Similar articles
-
Recent advances in the relationship between obesity, inflammation, and insulin resistance.Eur Cytokine Netw. 2006 Mar;17(1):4-12. Eur Cytokine Netw. 2006. PMID: 16613757 Review.
-
Decoding cell death signals in liver inflammation.J Hepatol. 2013 Sep;59(3):583-94. doi: 10.1016/j.jhep.2013.03.033. Epub 2013 Apr 6. J Hepatol. 2013. PMID: 23567086 Review.
-
Fats, inflammation and insulin resistance: insights to the role of macrophage and T-cell accumulation in adipose tissue.Proc Nutr Soc. 2011 Nov;70(4):408-17. doi: 10.1017/S0029665111000565. Epub 2011 Aug 12. Proc Nutr Soc. 2011. PMID: 21835098 Review.
-
The "Big Bang" in obese fat: Events initiating obesity-induced adipose tissue inflammation.Eur J Immunol. 2015 Sep;45(9):2446-56. doi: 10.1002/eji.201545502. Epub 2015 Aug 19. Eur J Immunol. 2015. PMID: 26220361 Review.
-
Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells.Vitam Horm. 2006;74:443-77. doi: 10.1016/S0083-6729(06)74018-3. Vitam Horm. 2006. PMID: 17027526 Review.
Cited by
-
Systemic Inflammation in Severe Obese Patients Undergoing Surgery for Obesity and Weight-Related Diseases.Obes Surg. 2018 Jul;28(7):1931-1942. doi: 10.1007/s11695-017-3104-9. Obes Surg. 2018. PMID: 29497960 Free PMC article. Clinical Trial.
-
Abscisic Acid as Pathogen Effector and Immune Regulator.Front Plant Sci. 2017 Apr 19;8:587. doi: 10.3389/fpls.2017.00587. eCollection 2017. Front Plant Sci. 2017. PMID: 28469630 Free PMC article. Review.
-
Molecular Mechanisms Underlying the Effects of Olive Oil Triterpenic Acids in Obesity and Related Diseases.Nutrients. 2022 Apr 12;14(8):1606. doi: 10.3390/nu14081606. Nutrients. 2022. PMID: 35458168 Free PMC article. Review.
-
Phenolic Compounds Known to Be Present in Lingonberry (Vaccinium vitis-idaea L.) Enhance Macrophage Polarization towards the Anti-Inflammatory M2 Phenotype.Biomedicines. 2022 Nov 25;10(12):3045. doi: 10.3390/biomedicines10123045. Biomedicines. 2022. PMID: 36551801 Free PMC article.
-
Transcriptional and Functional Plasticity Induced by Chronic Insulin Exposure in a Mast Cell-Like Basophilic Leukemia Cell Model.J Immunobiol. 2017;2(4):135. doi: 10.4172/2476-1966.1000135. Epub 2017 Dec 11. J Immunobiol. 2017. PMID: 29430572 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

