Influenza infection modulates vesicular trafficking and induces Golgi complex disruption
- PMID: 28004015
- PMCID: PMC5142599
- DOI: 10.1007/s13337-016-0347-3
Influenza infection modulates vesicular trafficking and induces Golgi complex disruption
Abstract
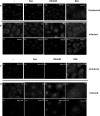
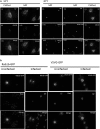
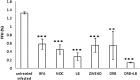
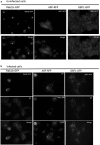
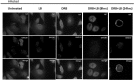
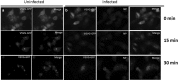
Influenza A virus (IFV) replicates its genome in the nucleus of infected cells and uses the cellular protein transport system for genome trafficking from the nucleus to the plasma membrane. However, many details of the mechanism of this process, and its relationship to subsequent cytoplasmic virus trafficking, have not been elucidated. We examined the effect of nuclear transport inhibitors Leptomycin B (LB), 5,6 dichloro-1-β-d-ribofuranosyl-benzimidazole (DRB), the vesicular transport inhibitor Brefeldin A (BFA), the caspase inhibitor ZWEHD, and microtubule inhibitor Nocodazole (NOC) on virus replication and intracellular trafficking of viral nucleoprotein (NP) from the nucleus to the ER and Golgi. Also, we carried out complementary studies to determine the effect of IFV on intracellular membranes. Inhibition of the CRM1 and TAP-P15 nuclear transport pathways by DRB and LB blocked completely the export of virus. Inhibition of vesicular trafficking by BFA, NOC, and ZWEHD also affected influenza infection. Interestingly, IFV infection induced fragmentation of the Golgi complex resulting in diffuse distribution of large and small vesicles throughout the cytoplasm. Live-cell microscopy revealed expansion of Golgi localization signals indicating progressive dispersion of Golgi positive structures, resulting in the disassembly of the Golgi ribbon structure. Other vesicular components (Rab1b, ARF1 and GBF1) were also found to be required for IFV infection. Furthermore, the exact step at which IFV infection disrupts vesicle trafficking was identified as the ER-Golgi intermediate compartment. These findings suggest that IFV NP is trafficked from the nucleus via the CRM1 and TAP pathways. IFV modulates vesicular trafficking inducing disruption of the Golgi complex. These studies provide insight on the ways in which IFV affects intracellular trafficking of different host proteins and will facilitate identification of useful pharmaceutical targets to abrogate virus replication.
Keywords: Golgi fragmentation; Influenza virus; Vesicular components.
Figures
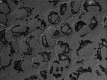
Similar articles
-
Rab1b-GBF1-ARFs mediated intracellular trafficking is required for classical swine fever virus replication in swine umbilical vein endothelial cells.Vet Microbiol. 2020 Jul;246:108743. doi: 10.1016/j.vetmic.2020.108743. Epub 2020 Jun 1. Vet Microbiol. 2020. PMID: 32605744
-
Poliovirus infection blocks ERGIC-to-Golgi trafficking and induces microtubule-dependent disruption of the Golgi complex.J Cell Sci. 2007 Sep 15;120(Pt 18):3207-18. doi: 10.1242/jcs.03483. Epub 2007 Aug 21. J Cell Sci. 2007. PMID: 17711878
-
Involvement of membrane traffic in the replication of poliovirus genomes: effects of brefeldin A.Virology. 1992 Nov;191(1):166-75. doi: 10.1016/0042-6822(92)90178-r. Virology. 1992. PMID: 1329315
-
TRP Channel Trafficking.In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 23. In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 23. PMID: 21204515 Free Books & Documents. Review.
-
Role of microtubules in the organization of the Golgi complex.Exp Cell Res. 1999 Feb 1;246(2):263-79. doi: 10.1006/excr.1998.4326. Exp Cell Res. 1999. PMID: 9925741 Review.
Cited by
-
CRISPR screens and lectin microarrays identify novel high mannose N-glycan regulators.bioRxiv [Preprint]. 2024 Apr 10:2023.10.23.563662. doi: 10.1101/2023.10.23.563662. bioRxiv. 2024. Update in: Nat Commun. 2024 Nov 18;15(1):9970. doi: 10.1038/s41467-024-53225-1 PMID: 37961200 Free PMC article. Updated. Preprint.
-
Porcine reproductive and respiratory syndrome virus triggers Golgi apparatus fragmentation-mediated autophagy to facilitate viral self-replication.J Virol. 2024 Feb 20;98(2):e0184223. doi: 10.1128/jvi.01842-23. Epub 2024 Jan 5. J Virol. 2024. PMID: 38179942 Free PMC article.
-
CRISPR screens and lectin microarrays identify high mannose N-glycan regulators.Nat Commun. 2024 Nov 18;15(1):9970. doi: 10.1038/s41467-024-53225-1. Nat Commun. 2024. PMID: 39557836 Free PMC article.
-
A Review and Meta-Analysis of Influenza Interactome Studies.Front Microbiol. 2022 Apr 21;13:869406. doi: 10.3389/fmicb.2022.869406. eCollection 2022. Front Microbiol. 2022. PMID: 35531276 Free PMC article. Review.
-
Canonical and Noncanonical Autophagy as Potential Targets for COVID-19.Cells. 2020 Jul 5;9(7):1619. doi: 10.3390/cells9071619. Cells. 2020. PMID: 32635598 Free PMC article. Review.
References
-
- Arcangeletti MC, Pinardi F, Missorini S, De Conto F, Conti G, Portincasa P, Scherrer K, Chezzi C. Modification of cytoskeleton and prosome networks in relation to protein synthesis in influenza A virus-infected LLC-MK2 cells. Virus Res. 1997;51:19–34. doi: 10.1016/S0168-1702(97)00074-9. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

