Ghrelin, CCK, GLP-1, and PYY(3-36): Secretory Controls and Physiological Roles in Eating and Glycemia in Health, Obesity, and After RYGB
- PMID: 28003328
- PMCID: PMC6151490
- DOI: 10.1152/physrev.00031.2014
Ghrelin, CCK, GLP-1, and PYY(3-36): Secretory Controls and Physiological Roles in Eating and Glycemia in Health, Obesity, and After RYGB
Erratum in
-
Corrigendum.Physiol Rev. 2017 Jul 1;97(3):1229. doi: 10.1152/physrev.z9j-2792-corr.2011. Physiol Rev. 2017. PMID: 28615464 Free PMC article. No abstract available.
Abstract
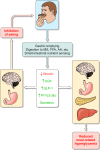
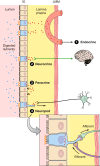
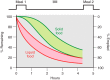
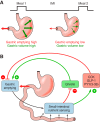
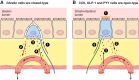
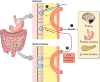
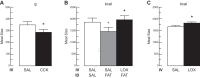
The efficacy of Roux-en-Y gastric-bypass (RYGB) and other bariatric surgeries in the management of obesity and type 2 diabetes mellitus and novel developments in gastrointestinal (GI) endocrinology have renewed interest in the roles of GI hormones in the control of eating, meal-related glycemia, and obesity. Here we review the nutrient-sensing mechanisms that control the secretion of four of these hormones, ghrelin, cholecystokinin (CCK), glucagon-like peptide-1 (GLP-1), and peptide tyrosine tyrosine [PYY(3-36)], and their contributions to the controls of GI motor function, food intake, and meal-related increases in glycemia in healthy-weight and obese persons, as well as in RYGB patients. Their physiological roles as classical endocrine and as locally acting signals are discussed. Gastric emptying, the detection of specific digestive products by small intestinal enteroendocrine cells, and synergistic interactions among different GI loci all contribute to the secretion of ghrelin, CCK, GLP-1, and PYY(3-36). While CCK has been fully established as an endogenous endocrine control of eating in healthy-weight persons, the roles of all four hormones in eating in obese persons and following RYGB are uncertain. Similarly, only GLP-1 clearly contributes to the endocrine control of meal-related glycemia. It is likely that local signaling is involved in these hormones' actions, but methods to determine the physiological status of local signaling effects are lacking. Further research and fresh approaches are required to better understand ghrelin, CCK, GLP-1, and PYY(3-36) physiology; their roles in obesity and bariatric surgery; and their therapeutic potentials.
Copyright © 2017 the American Physiological Society.
Figures
Similar articles
-
Effect of Roux-en-Y gastric bypass on the distribution and hormone expression of small-intestinal enteroendocrine cells in obese patients with type 2 diabetes.Diabetologia. 2015 Oct;58(10):2254-8. doi: 10.1007/s00125-015-3696-3. Epub 2015 Jul 18. Diabetologia. 2015. PMID: 26186884
-
Changes in GI hormones and their effect on gastric emptying and transit times after Roux-en-Y gastric bypass in rat model.Surgery. 2005 Aug;138(2):283-90. doi: 10.1016/j.surg.2005.05.013. Surgery. 2005. PMID: 16153438
-
Ghrelin suppresses cholecystokinin (CCK), peptide YY (PYY) and glucagon-like peptide-1 (GLP-1) in the intestine, and attenuates the anorectic effects of CCK, PYY and GLP-1 in goldfish (Carassius auratus).Horm Behav. 2017 Jul;93:62-71. doi: 10.1016/j.yhbeh.2017.05.004. Epub 2017 May 18. Horm Behav. 2017. PMID: 28506816
-
EndoBarrier gastrointestinal liner. Delineation of underlying mechanisms and clinical effects.Dan Med J. 2016 Nov;63(11):B5309. Dan Med J. 2016. PMID: 27808040 Review.
-
Mucosal and hormonal adaptations after Roux-en-Y gastric bypass.Surg Obes Relat Dis. 2023 Jan;19(1):37-49. doi: 10.1016/j.soard.2022.08.020. Epub 2022 Sep 13. Surg Obes Relat Dis. 2023. PMID: 36243547 Free PMC article. Review.
Cited by
-
The Role of GLP-1, GIP, MCP-1 and IGFBP-7 Biomarkers in the Development of Metabolic Disorders: A Review and Predictive Analysis in the Context of Diabetes and Obesity.Biomedicines. 2024 Jan 11;12(1):159. doi: 10.3390/biomedicines12010159. Biomedicines. 2024. PMID: 38255264 Free PMC article. Review.
-
Mechanism of Takifugu bimaculatus Skin Peptides in Alleviating Hyperglycemia in Rats with Type 2 Diabetic Mellitus Based on Microbiome and Metabolome Analyses.Mar Drugs. 2024 Aug 22;22(8):377. doi: 10.3390/md22080377. Mar Drugs. 2024. PMID: 39195493 Free PMC article.
-
G protein-coupled receptors and obesity.Front Endocrinol (Lausanne). 2023 Dec 14;14:1301017. doi: 10.3389/fendo.2023.1301017. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38161982 Free PMC article. Review.
-
Glucocorticoids, stress and eating: The mediating role of appetite-regulating hormones.Obes Rev. 2023 Mar;24(3):e13539. doi: 10.1111/obr.13539. Epub 2022 Dec 8. Obes Rev. 2023. PMID: 36480471 Free PMC article. Review.
-
Acute Effects of Substitution, and Addition, of Carbohydrates and Fat to Protein on Gastric Emptying, Blood Glucose, Gut Hormones, Appetite, and Energy Intake.Nutrients. 2018 Oct 7;10(10):1451. doi: 10.3390/nu10101451. Nutrients. 2018. PMID: 30301241 Free PMC article. Clinical Trial.
References
-
- Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR. The inhibitory effects of peripheral administration of peptide YY(3–36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res : 127–131, 2005. - PubMed
-
- Abbott CR, Small CJ, Kennedy AR, Neary NM, Sajedi A, Ghatei MA, Bloom SR. Blockade of the neuropeptide Y Y2 receptor with the specific antagonist BIIE0246 attenuates the effect of endogenous and exogenous peptide YY(3–36) on food intake. Brain Res : 139–144, 2005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

