Apoptosis in Cellular Society: Communication between Apoptotic Cells and Their Neighbors
- PMID: 27999411
- PMCID: PMC5187944
- DOI: 10.3390/ijms17122144
Apoptosis in Cellular Society: Communication between Apoptotic Cells and Their Neighbors
Abstract
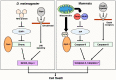
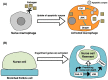
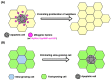
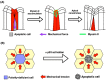
Apoptosis is one of the cell-intrinsic suicide programs and is an essential cellular behavior for animal development and homeostasis. Traditionally, apoptosis has been regarded as a cell-autonomous phenomenon. However, recent in vivo genetic studies have revealed that apoptotic cells actively influence the behaviors of surrounding cells, including engulfment, proliferation, and production of mechanical forces. Such interactions can be bidirectional, and apoptosis is non-autonomously induced in a cellular community. Of note, it is becoming evident that active communication between apoptotic cells and living cells contributes to physiological processes during tissue remodeling, regeneration, and morphogenesis. In this review, we focus on the mutual interactions between apoptotic cells and their neighbors in cellular society and discuss issues relevant to future studies of apoptosis.
Keywords: apoptosis; engulfment; mechanical force; non-cell autonomous effects; proliferation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Spreading the word: non-autonomous effects of apoptosis during development, regeneration and disease.Development. 2015 Oct 1;142(19):3253-62. doi: 10.1242/dev.127878. Development. 2015. PMID: 26443630 Free PMC article. Review.
-
Mechanisms and Significance of Phagocytic Elimination of Cells Undergoing Apoptotic Death.Biol Pharm Bull. 2017;40(11):1819-1827. doi: 10.1248/bpb.b17-00478. Biol Pharm Bull. 2017. PMID: 29093328 Review.
-
Phagocytosis of apoptotic cells in mammals, caenorhabditis elegans and Drosophila melanogaster: molecular mechanisms and physiological consequences.Front Biosci. 2002 May 1;7:d1298-313. doi: 10.2741/A841. Front Biosci. 2002. PMID: 11991836 Review.
-
The genetics of hiding the corpse: engulfment and degradation of apoptotic cells in C. elegans and D. melanogaster.Curr Top Dev Biol. 2004;63:91-143. doi: 10.1016/S0070-2153(04)63004-3. Curr Top Dev Biol. 2004. PMID: 15536015 Review. No abstract available.
-
How winner cells cause the demise of loser cells: cell competition causes apoptosis of suboptimal cells: their dregs are removed by hemocytes, thus preserving tissue homeostasis.Bioessays. 2013 Apr;35(4):348-53. doi: 10.1002/bies.201200156. Epub 2013 Feb 4. Bioessays. 2013. PMID: 23382037
Cited by
-
Cell Death, by Any Other Name….Cells. 2024 Feb 10;13(4):325. doi: 10.3390/cells13040325. Cells. 2024. PMID: 38391938 Free PMC article. Review.
-
Identification of TNFRSF21 as an inhibitory factor of osteosarcoma based on a necroptosis-related prognostic gene signature and molecular experiments.Cancer Cell Int. 2024 Jan 6;24(1):14. doi: 10.1186/s12935-023-03198-w. Cancer Cell Int. 2024. PMID: 38184626 Free PMC article.
-
RNA modifications act as regulators of cell death.RNA Biol. 2021 Dec;18(12):2183-2193. doi: 10.1080/15476286.2021.1925460. Epub 2021 Jul 27. RNA Biol. 2021. PMID: 34313542 Free PMC article. Review.
-
Preparation of kakkatin derivatives and their anti-tumor activity.World J Clin Oncol. 2024 Aug 24;15(8):1078-1091. doi: 10.5306/wjco.v15.i8.1078. World J Clin Oncol. 2024. PMID: 39193163 Free PMC article.
-
Clinical efficacy of intermittent magnetic pressure therapy for ear keloid treatment after excision.Arch Craniofac Surg. 2019 Dec;20(6):354-360. doi: 10.7181/acfs.2019.00465. Epub 2019 Dec 20. Arch Craniofac Surg. 2019. PMID: 31914489 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

