Exercise therapy for chronic fatigue syndrome
- PMID: 27995604
- PMCID: PMC6473631
- DOI: 10.1002/14651858.CD003200.pub6
Exercise therapy for chronic fatigue syndrome
Update in
-
Exercise therapy for chronic fatigue syndrome.Cochrane Database Syst Rev. 2017 Apr 25;4(4):CD003200. doi: 10.1002/14651858.CD003200.pub7. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2019 Oct 02;10:CD003200. doi: 10.1002/14651858.CD003200.pub8 PMID: 28444695 Free PMC article. Updated. Review.
Abstract
Background: Chronic fatigue syndrome (CFS) is characterised by persistent, medically unexplained fatigue, as well as symptoms such as musculoskeletal pain, sleep disturbance, headaches and impaired concentration and short-term memory. CFS presents as a common, debilitating and serious health problem. Treatment may include physical interventions, such as exercise therapy, which was last reviewed in 2004.
Objectives: The objective of this review was to determine the effects of exercise therapy (ET) for patients with CFS as compared with any other intervention or control.• Exercise therapy versus 'passive control' (e.g. treatment as usual, waiting-list control, relaxation, flexibility).• Exercise therapy versus other active treatment (e.g. cognitive-behavioural therapy (CBT), cognitive treatment, supportive therapy, pacing, pharmacological therapy such as antidepressants).• Exercise therapy in combination with other specified treatment strategies versus other specified treatment strategies (e.g. exercise combined with pharmacological treatment vs pharmacological treatment alone).
Search methods: We searched The Cochrane Collaboration Depression, Anxiety and Neurosis Controlled Trials Register (CCDANCTR), the Cochrane Central Register of Controlled Trials (CENTRAL) and SPORTDiscus up to May 2014 using a comprehensive list of free-text terms for CFS and exercise. We located unpublished or ongoing trials through the World Health Organization (WHO) International Clinical Trials Registry Platform (to May 2014). We screened reference lists of retrieved articles and contacted experts in the field for additional studies SELECTION CRITERIA: Randomised controlled trials involving adults with a primary diagnosis of CFS who were able to participate in exercise therapy. Studies had to compare exercise therapy with passive control, psychological therapies, adaptive pacing therapy or pharmacological therapy.
Data collection and analysis: Two review authors independently performed study selection, risk of bias assessments and data extraction. We combined continuous measures of outcomes using mean differences (MDs) and standardised mean differences (SMDs). We combined serious adverse reactions and drop-outs using risk ratios (RRs). We calculated an overall effect size with 95% confidence intervals (CIs) for each outcome.
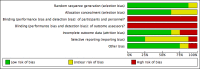
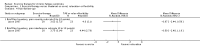
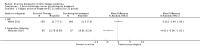
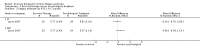
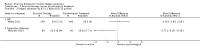
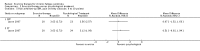
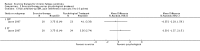
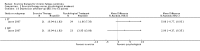
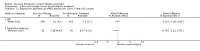
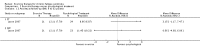
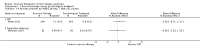
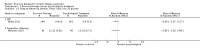
Main results: We have included eight randomised controlled studies and have reported data from 1518 participants in this review. Three studies diagnosed individuals with CFS using the 1994 criteria of the Centers for Disease Control and Prevention (CDC); five used the Oxford criteria. Exercise therapy lasted from 12 to 26 weeks. Seven studies used variations of aerobic exercise therapy such as walking, swimming, cycling or dancing provided at mixed levels in terms of intensity of the aerobic exercise from very low to quite rigorous, whilst one study used anaerobic exercise. Control groups consisted of passive control (eight studies; e.g. treatment as usual, relaxation, flexibility) or CBT (two studies), cognitive therapy (one study), supportive listening (one study), pacing (one study), pharmacological treatment (one study) and combination treatment (one study). Risk of bias varied across studies, but within each study, little variation was found in the risk of bias across our primary and secondary outcome measures.Investigators compared exercise therapy with 'passive' control in eight trials, which enrolled 971 participants. Seven studies consistently showed a reduction in fatigue following exercise therapy at end of treatment, even though the fatigue scales used different scoring systems: an 11-item scale with a scoring system of 0 to 11 points (MD -6.06, 95% CI -6.95 to -5.17; one study, 148 participants; low-quality evidence); the same 11-item scale with a scoring system of 0 to 33 points (MD -2.82, 95% CI -4.07 to -1.57; three studies, 540 participants; moderate-quality evidence); and a 14-item scale with a scoring system of 0 to 42 points (MD -6.80, 95% CI -10.31 to -3.28; three studies, 152 participants; moderate-quality evidence). Serious adverse reactions were rare in both groups (RR 0.99, 95% CI 0.14 to 6.97; one study, 319 participants; moderate-quality evidence), but sparse data made it impossible for review authors to draw conclusions. Study authors reported a positive effect of exercise therapy at end of treatment with respect to sleep (MD -1.49, 95% CI -2.95 to -0.02; two studies, 323 participants), physical functioning (MD 13.10, 95% CI 1.98 to 24.22; five studies, 725 participants) and self-perceived changes in overall health (RR 1.83, 95% CI 1.39 to 2.40; four studies, 489 participants). It was not possible for review authors to draw conclusions regarding the remaining outcomes.Investigators compared exercise therapy with CBT in two trials (351 participants). One trial (298 participants) reported little or no difference in fatigue at end of treatment between the two groups using an 11-item scale with a scoring system of 0 to 33 points (MD 0.20, 95% CI -1.49 to 1.89). Both studies measured differences in fatigue at follow-up, but neither found differences between the two groups using an 11-item fatigue scale with a scoring system of 0 to 33 points (MD 0.30, 95% CI -1.45 to 2.05) and a nine-item Fatigue Severity Scale with a scoring system of 1 to 7 points (MD 0.40, 95% CI -0.34 to 1.14). Serious adverse reactions were rare in both groups (RR 0.67, 95% CI 0.11 to 3.96). We observed little or no difference in physical functioning, depression, anxiety and sleep, and we were not able to draw any conclusions with regard to pain, self-perceived changes in overall health, use of health service resources and drop-out rate.With regard to other comparisons, one study (320 participants) suggested a general benefit of exercise over adaptive pacing, and another study (183 participants) a benefit of exercise over supportive listening. The available evidence was too sparse to draw conclusions about the effect of pharmaceutical interventions.
Authors' conclusions: Patients with CFS may generally benefit and feel less fatigued following exercise therapy, and no evidence suggests that exercise therapy may worsen outcomes. A positive effect with respect to sleep, physical function and self-perceived general health has been observed, but no conclusions for the outcomes of pain, quality of life, anxiety, depression, drop-out rate and health service resources were possible. The effectiveness of exercise therapy seems greater than that of pacing but similar to that of CBT. Randomised trials with low risk of bias are needed to investigate the type, duration and intensity of the most beneficial exercise intervention.
Conflict of interest statement
LL: nothing to declare. KGB: nothing to declare. JO‐J: nothing to declare. JRP: nothing to declare.
Figures
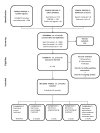






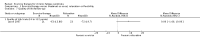
















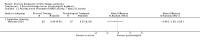
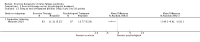











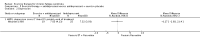
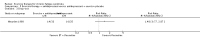
Update of
-
Exercise therapy for chronic fatigue syndrome.Cochrane Database Syst Rev. 2016 Jun 24;(6):CD003200. doi: 10.1002/14651858.CD003200.pub5. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2016 Dec 20;12:CD003200. doi: 10.1002/14651858.CD003200.pub6 PMID: 27339435 Updated. Review.
Similar articles
-
Exercise therapy for chronic fatigue syndrome.Cochrane Database Syst Rev. 2015 Feb 10;(2):CD003200. doi: 10.1002/14651858.CD003200.pub3. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2016 Feb 07;2:CD003200. doi: 10.1002/14651858.CD003200.pub4 PMID: 25674924 Updated. Review.
-
Exercise therapy for chronic fatigue syndrome.Cochrane Database Syst Rev. 2017 Apr 25;4(4):CD003200. doi: 10.1002/14651858.CD003200.pub7. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2019 Oct 02;10:CD003200. doi: 10.1002/14651858.CD003200.pub8 PMID: 28444695 Free PMC article. Updated. Review.
-
Exercise therapy for chronic fatigue syndrome.Cochrane Database Syst Rev. 2016 Jun 24;(6):CD003200. doi: 10.1002/14651858.CD003200.pub5. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2016 Dec 20;12:CD003200. doi: 10.1002/14651858.CD003200.pub6 PMID: 27339435 Updated. Review.
-
Exercise therapy for chronic fatigue syndrome.Cochrane Database Syst Rev. 2016 Feb 7;2:CD003200. doi: 10.1002/14651858.CD003200.pub4. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2016 Jun 24;(6):CD003200. doi: 10.1002/14651858.CD003200.pub5 PMID: 26852189 Updated. Review.
-
Non-pharmacological interventions for somatoform disorders and medically unexplained physical symptoms (MUPS) in adults.Cochrane Database Syst Rev. 2014 Nov 1;2014(11):CD011142. doi: 10.1002/14651858.CD011142.pub2. Cochrane Database Syst Rev. 2014. PMID: 25362239 Free PMC article. Review.
Cited by
-
The Influence of Branched-Chain Amino Acid Supplementation on Fatigue and Tryptophan Metabolism After Acute and Chronic Exercise in Older Adults: Protocol for a Pilot Randomized Controlled Trial.JMIR Res Protoc. 2023 Nov 1;12:e52199. doi: 10.2196/52199. JMIR Res Protoc. 2023. PMID: 37910166 Free PMC article.
-
Effects of an integrative multimodal inpatient program on fatigue and work ability in patients with Post-COVID Syndrome-a prospective observational study.Eur Arch Psychiatry Clin Neurosci. 2024 Dec;274(8):1983-1991. doi: 10.1007/s00406-024-01792-1. Epub 2024 Apr 5. Eur Arch Psychiatry Clin Neurosci. 2024. PMID: 38578435
-
The Impact of a Structured Exercise Programme upon Cognitive Function in Chronic Fatigue Syndrome Patients.Brain Sci. 2019 Dec 19;10(1):4. doi: 10.3390/brainsci10010004. Brain Sci. 2019. PMID: 31861543 Free PMC article.
-
Chronic Fatigue Syndrome prevalence is grossly overestimated using Oxford criteria compared to Centers for Disease Control (Fukuda) criteria in a U.S. population study.Fatigue. 2017;5(4):215-230. doi: 10.1080/21641846.2017.1353578. Epub 2017 Jul 21. Fatigue. 2017. PMID: 30854252 Free PMC article.
-
Conduct and reporting of citation searching in Cochrane systematic reviews: A cross-sectional study.Res Synth Methods. 2020 Mar;11(2):169-180. doi: 10.1002/jrsm.1355. Epub 2019 Jul 4. Res Synth Methods. 2020. PMID: 31127978 Free PMC article.
References
References to studies included in this review
-
- Fulcher KY, White PD. Chronic fatigue syndrome: a description of graded exercise treatment. Physiotherapy 1998;84(9):223‐6. []
- Fulcher KY, White PD. Randomised controlled trial of graded exercise in patients with chronic fatigue syndrome. BMJ 1997;314(7095):1647‐52. [] - PMC - PubMed
- White PD, Fulcher KY. A randomised controlled trial of graded exercise in patients with a chronic fatigue. Royal College of Psychiatrists Winter Meeting, Cardiff. 1997. [] - PMC - PubMed
-
2892995
-
- Hlavaty LE, Brown MM, Jason LA. The effect of homework compliance on treatment outcomes for participants with myalgic encephalomyelitis/chronic fatigue syndrome. Rehabilitation Psychology 2011;56(3):212‐8. [] - PMC - PubMed
- Jason L, Torres‐Harding S, Friedberg F, Corradi K, Njoku M Donalek J, et al. Non‐pharmacologic interventions for CFS: a randomized trial. Journal of Clinical Psychology in Medical Settings 2007;172:485‐90. []
-
2892999
-
- Moss‐Morriss R, Sharon C, Tobin R, Baldi JC. A randomized controlled graded exercise trial for chronic fatigue syndrome: outcomes and mechanisms of change. Journal of Health Psychology 2005;10(2):245‐59. [] - PubMed
-
2893002
-
- Powell P, Bentall ROP, Nye FJ, Edwards RHT. Patient education to encourage graded exercise in chronic fatigue syndrome: 2‐year follow‐up of randomised controlled trial. The British Journal of Psychiatry 2004;184:142‐6. [] - PubMed
- Powell P, Bentall RP, Nye FJ, Edwards RH. Randomised controlled trial of patient education to encourage graded exercise in chronic fatigue syndrome. BMJ 2001;322(7283):387‐90. [] - PMC - PubMed
-
2893004
-
- Wallman KE, Morton AR, Goodman C, Grove R. Exercise prescription for individuals with chronic fatigue syndrome. Medical Journal of Australia 2005;183(3):142‐3. [] - PubMed
- Wallman KE, Morton AR, Goodman C, Grove R, Guilfoyle AM. Randomised controlled trial of graded exercise in chronic fatigue syndrome. Medical Journal of Australia 2004;180(9):444‐8. [] - PubMed
-
2893007
References to studies excluded from this review
-
- Evering RMH. Ambulatory feedback at daily physical activity patterns. A treatment for the chronic fatigue syndrome in the home environment?. Universitet Twente, Netherlands2013:1‐223. []
- Evering RMH. Optimalization of cognitive behavioral therapy (CBT) for CFS patients in rehabilitation by means of ambulatory activity‐based feedback (ABF). trialregister.nl/trialreg/admin/rctview.asp?TC=1513 (accessed 7 May 2013). []
-
2893027
-
- Guarino P, Peduzzi P, Donta ST, Engel CC Jr, Clauw DJ, Williams DA, et al. A multicenter two by two factorial trial of cognitive behavioral therapy and aerobic exercise for gulf war veterans' illnesses: design of a Veterans Affairs cooperative study (CSP #470). Controlled Clinical Trials 2001;22:31032. [] - PubMed
-
2893032
-
- Nunez M, Fernandez Soles J, Nunez E, Fernandez Huerta JM, Godas Sieso T, Gomez Gil E. Health‐related quality of life in patients with chronic fatigue syndrome: group cognitive behavioural therapy and graded exercise versus usual treatment. A randomised controlled trial with 1 year of follow‐up. Clinical Rheumatology 2011;30(3):381‐9. [] - PubMed
-
2893034
-
- Risdale L, Darbishire L, Seed T. Is graded exercise better than cognitive behaviour therapy for fatigue? A UK randomized trial in primary care. Psychological Medicine 2003;34:37‐49. [] - PubMed
-
2893036
References to studies awaiting assessment
-
- Hatcher S. A randomised double‐blind placebo controlled trial of dothiepin and graded activity in the treatment of chronic fatigue syndrome. Personal communication, 1998. []
-
2893059
-
- Liu CZ, Lei B. Effect of Tuina on oxygen free radicals metabolism in patients with chronic fatigue syndrome [Chinese]. Zhongguo Zhenjiu 2010;11:946‐8. [] - PubMed
-
2893061
-
- Zhuo J‐X, Gu L‐Y. Relative research on treating chronic fatigue syndrome with gradual exercise. Journal of Beijing Sport University 2007;30(6):801‐3. []
-
2893063
References to ongoing studies
-
- Broadbent S, Coutts R. The protocol for a randomised controlled trial comparing intermittent and graded exercise to usual care for chronic fatigue syndrome patients. BMC Sports Science, Medicine & Rehabilitation 2013;5(1):1‐6. [] - PMC - PubMed
- Broadbent S. A pilot study on the effects of intermittent and graded exercise compared to no exercise for optimising health and reducing symptoms in chronic fatigue syndrome (CFS) patients. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12612001241820 (accessed 7 May 2013). []
-
2893065
-
- Kos D, Nijs J. Pacing activity self‐management for patients with chronic fatigue syndrome: randomized controlled clinical trial, 2012. clinicaltrials.gov/show/NCT01512342 (accessed 7 May 2013). []
-
2893068
-
- Marques M, Gucht V, Maes S, Leal I. Protocol for the "four steps to control your fatigue (4‐STEPS)" randomised controlled trial: a self‐regulation based physical activity intervention for patients with unexplained chronic fatigue. BMC Public Health 2012;12:202. [; DOI: 10.1186/1471-2458-12-202] - DOI - PMC - PubMed
-
2893070
-
- Vos‐Vromans D. Is a multidisciplinary rehabilitation treatment more effective than mono disciplinary cognitive behavioural therapy for patients with chronic fatigue syndrome? A multi centre randomised controlled trial [FatiGo, ISRCTN77567702]. http://www.controlled‐trials.com/isrctn/pf/77567702 (accessed 7 May 2013). [; ISRCTN77567702 ]
- Vos‐Vromans DCWM, Smeets RJEM, Rijnders LJM, Gorrissen RRM, Pont M, Köke AJA, et al. Cognitive behavioural therapy versus multidisciplinary rehabilitation treatment for patients with chronic fatigue syndrome: study protocol for a randomized controlled trial (FatiGo). Trials [electronic resource] 2012;13:71. [] - PMC - PubMed
-
2893072
-
- White PD. Therapy guided self‐help treatment (GETSET) for patients with chronic fatigue syndrome/myalgic encephalomyelitis: a randomised controlled trial in secondary care. ISRCTN22975026, 2012. http://www.controlled‐trials.com/ISRCTN22975026/GETSET (accessed 30 Octrober 2014). []
-
2893075
Additional references
-
- American College of Sports Medicine. ACSM`s Resource Manual for Guidelines for Exercise Testing and Prescription. 4th Edition. Baltimore, MD: Lippincott Williams & Wilkins, 2001.
-
- Alderson P, Green S, Higgins JP, editors. Cochrane Reviewers’ Handbook 4.2.2 [updated December 2003]. The Cochrane Library, Issue 1. Chichester, UK: John Wiley & Sons Ltd, 2004.
-
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory‐II. Manual for the Beck Depression Inventory‐II. San Antonio: Psychological Cooperation, 1996.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

