Epigallocatechin-3-Gallate Accelerates Relaxation and Ca2+ Transient Decay and Desensitizes Myofilaments in Healthy and Mybpc3-Targeted Knock-in Cardiomyopathic Mice
- PMID: 27994558
- PMCID: PMC5136558
- DOI: 10.3389/fphys.2016.00607
Epigallocatechin-3-Gallate Accelerates Relaxation and Ca2+ Transient Decay and Desensitizes Myofilaments in Healthy and Mybpc3-Targeted Knock-in Cardiomyopathic Mice
Abstract
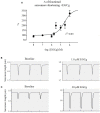
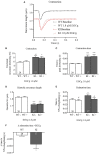
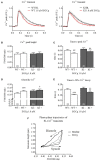
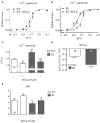
Background: Hypertrophic cardiomyopathy (HCM) is the most common inherited cardiac muscle disease with left ventricular hypertrophy, interstitial fibrosis and diastolic dysfunction. Increased myofilament Ca2+ sensitivity could be the underlying cause of diastolic dysfunction. Epigallocatechin-3-gallate (EGCg), a catechin found in green tea, has been reported to decrease myofilament Ca2+ sensitivity in HCM models with troponin mutations. However, whether this is also the case for HCM-associated thick filament mutations is not known. Therefore, we evaluated whether EGCg affects the behavior of cardiomyocytes and myofilaments of an HCM mouse model carrying a gene mutation in cardiac myosin-binding protein C and exhibiting both increased myofilament Ca2+ sensitivity and diastolic dysfunction. Methods and Results: Acute effects of EGCg were tested on fractional sarcomere shortening and Ca2+ transients in intact ventricular myocytes and on force-Ca2+ relationship of skinned ventricular muscle strips isolated from Mybpc3-targeted knock-in (KI) and wild-type (WT) mice. Fractional sarcomere shortening and Ca2+ transients were analyzed at 37°C under 1-Hz pacing in the absence or presence of EGCg (1.8 μM). At baseline and in the absence of Fura-2, KI cardiomyocytes displayed lower diastolic sarcomere length, higher fractional sarcomere shortening, longer time to peak shortening and time to 50% relengthening than WT cardiomyocytes. In WT and KI neither diastolic sarcomere length nor fractional sarcomere shortening were influenced by EGCg treatment, but relaxation time was reduced, to a greater extent in KI cells. EGCg shortened time to peak Ca2+ and Ca2+ transient decay in Fura-2-loaded WT and KI cardiomyocytes. EGCg did not influence phosphorylation of phospholamban. In skinned cardiac muscle strips, EGCg (30 μM) decreased Ca2+ sensitivity in both groups. Conclusion: EGCg hastened relaxation and Ca2+ transient decay to a larger extent in KI than in WT cardiomyocytes. This effect could be partially explained by myofilament Ca2+ desensitization.
Keywords: Ca2+ transient; Mybpc3; epigallocatechin-3-gallate; hypertrophic cardiomyopathy; myofilament Ca2+ sensitivity; relaxation.
Figures
Similar articles
-
Nebivolol Desensitizes Myofilaments of a Hypertrophic Cardiomyopathy Mouse Model.Front Physiol. 2017 Aug 2;8:558. doi: 10.3389/fphys.2017.00558. eCollection 2017. Front Physiol. 2017. PMID: 28824454 Free PMC article.
-
Diltiazem prevents stress-induced contractile deficits in cardiomyocytes, but does not reverse the cardiomyopathy phenotype in Mybpc3-knock-in mice.J Physiol. 2017 Jun 15;595(12):3987-3999. doi: 10.1113/JP273769. Epub 2017 Feb 7. J Physiol. 2017. PMID: 28090637 Free PMC article.
-
Increased myofilament Ca2+ sensitivity and diastolic dysfunction as early consequences of Mybpc3 mutation in heterozygous knock-in mice.J Mol Cell Cardiol. 2012 Jun;52(6):1299-307. doi: 10.1016/j.yjmcc.2012.03.009. Epub 2012 Mar 23. J Mol Cell Cardiol. 2012. PMID: 22465693 Free PMC article.
-
Cardiac myosin-binding protein C (MYBPC3) in cardiac pathophysiology.Gene. 2015 Dec 1;573(2):188-97. doi: 10.1016/j.gene.2015.09.008. Epub 2015 Sep 8. Gene. 2015. PMID: 26358504 Free PMC article. Review.
-
Investigating the role of uncoupling of troponin I phosphorylation from changes in myofibrillar Ca(2+)-sensitivity in the pathogenesis of cardiomyopathy.Front Physiol. 2014 Aug 25;5:315. doi: 10.3389/fphys.2014.00315. eCollection 2014. Front Physiol. 2014. PMID: 25202278 Free PMC article. Review.
Cited by
-
A comprehensive guide to genetic variants and post-translational modifications of cardiac troponin C.J Muscle Res Cell Motil. 2021 Jun;42(2):323-342. doi: 10.1007/s10974-020-09592-5. Epub 2020 Nov 11. J Muscle Res Cell Motil. 2021. PMID: 33179204 Free PMC article.
-
Molecular Defects in Cardiac Myofilament Ca2+-Regulation Due to Cardiomyopathy-Linked Mutations Can Be Reversed by Small Molecules Binding to Troponin.Front Physiol. 2018 Mar 27;9:243. doi: 10.3389/fphys.2018.00243. eCollection 2018. Front Physiol. 2018. PMID: 29636697 Free PMC article.
-
Green tea extract catechin improves cardiac function in pediatric cardiomyopathy patients with diastolic dysfunction.J Biomed Sci. 2019 May 8;26(1):32. doi: 10.1186/s12929-019-0528-7. J Biomed Sci. 2019. PMID: 31064352 Free PMC article.
-
Disease modeling of a mutation in α-actinin 2 guides clinical therapy in hypertrophic cardiomyopathy.EMBO Mol Med. 2019 Dec;11(12):e11115. doi: 10.15252/emmm.201911115. Epub 2019 Nov 3. EMBO Mol Med. 2019. PMID: 31680489 Free PMC article.
-
Pushing the Limits of Medical Management in HCM: A Review of Current Pharmacological Therapy Options.Int J Mol Sci. 2021 Jul 5;22(13):7218. doi: 10.3390/ijms22137218. Int J Mol Sci. 2021. PMID: 34281272 Free PMC article. Review.
References
-
- Alves M. L., Dias F. A., Gaffin R. D., Simon J. N., Montminy E. M., Biesiadecki B. J., et al. . (2014). Desensitization of myofilaments to Ca2+ as a therapeutic target for hypertrophic cardiomyopathy with mutations in thin filament proteins. Circ. Cardiovasc. Genet. 7, 132–143. 10.1161/CIRCGENETICS.113.000324 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

