Exosomes Derived from Human Endothelial Progenitor Cells Accelerate Cutaneous Wound Healing by Promoting Angiogenesis Through Erk1/2 Signaling
- PMID: 27994512
- PMCID: PMC5166489
- DOI: 10.7150/ijbs.15514
Exosomes Derived from Human Endothelial Progenitor Cells Accelerate Cutaneous Wound Healing by Promoting Angiogenesis Through Erk1/2 Signaling
Abstract
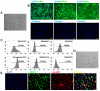
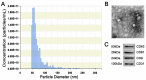
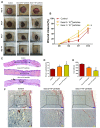
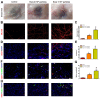
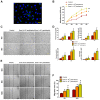
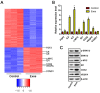
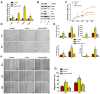
Chronic skin wounds represent one of the most common and disabling complications of diabetes. Endothelial progenitor cells (EPCs) are precursors of endothelial cells and can enhance diabetic wound repair by facilitating neovascularization. Recent studies indicate that the transplanted cells exert therapeutic effects primarily via a paracrine mechanism and exosomes are an important paracrine factor that can be directly used as therapeutic agents for regenerative medicine. However, application of exosomes in diabetic wound repair has been rarely reported. In this study, we demonstrated that the exosomes derived from human umbilical cord blood-derived EPCs (EPC-Exos) possessed robust pro-angiogenic and wound healing effects in streptozotocin-induced diabetic rats. By using a series of in vitro functional assays, we found that EPC-Exos could be incorporated into endothelial cells and significantly enhance endothelial cells' proliferation, migration, and angiogenic tubule formation. Moreover, microarray analyses indicated that exosomes treatment markedly altered the expression of a class of genes involved in Erk1/2 signaling pathway. It was further confirmed with functional study that this signaling process was the critical mediator during the exosomes-induced angiogenic responses of endothelial cells. Therefore, EPC-Exos are able to stimulate angiogenic activities of endothelial cells by activating Erk1/2 signaling, which finally facilitates cutaneous wound repair and regeneration.
Keywords: Angiogenesis; Erk1/2.; Exosomes; Microarray; Wound healing.
Conflict of interest statement
The authors have declared that no competing interest exists.
Figures
Similar articles
-
Human endothelial progenitor cells-derived exosomes accelerate cutaneous wound healing in diabetic rats by promoting endothelial function.J Diabetes Complications. 2016 Aug;30(6):986-92. doi: 10.1016/j.jdiacomp.2016.05.009. Epub 2016 May 13. J Diabetes Complications. 2016. PMID: 27236748
-
Exosomes secreted by endothelial progenitor cells accelerate bone regeneration during distraction osteogenesis by stimulating angiogenesis.Stem Cell Res Ther. 2019 Jan 11;10(1):12. doi: 10.1186/s13287-018-1115-7. Stem Cell Res Ther. 2019. PMID: 30635031 Free PMC article.
-
Exosomal DMBT1 from human urine-derived stem cells facilitates diabetic wound repair by promoting angiogenesis.Theranostics. 2018 Feb 7;8(6):1607-1623. doi: 10.7150/thno.22958. eCollection 2018. Theranostics. 2018. PMID: 29556344 Free PMC article.
-
[Research advances on the roles of exosomes derived from vascular endothelial progenitor cells in wound repair].Zhonghua Shao Shang Za Zhi. 2020 Sep 20;36(9):883-886. doi: 10.3760/cma.j.cn501120-20190702-00290. Zhonghua Shao Shang Za Zhi. 2020. PMID: 32972078 Review. Chinese.
-
Prospective application of exosomes derived from adipose-derived stem cells in skin wound healing: A review.J Cosmet Dermatol. 2020 Mar;19(3):574-581. doi: 10.1111/jocd.13215. Epub 2019 Nov 21. J Cosmet Dermatol. 2020. PMID: 31755172 Review.
Cited by
-
In-situ-sprayed therapeutic hydrogel for oxygen-actuated Janus regulation of postsurgical tumor recurrence/metastasis and wound healing.Nat Commun. 2024 Jan 27;15(1):814. doi: 10.1038/s41467-024-45072-x. Nat Commun. 2024. PMID: 38280861 Free PMC article.
-
Extracellular vesicle-enclosed miR-486-5p mediates wound healing with adipose-derived stem cells by promoting angiogenesis.J Cell Mol Med. 2020 Sep;24(17):9590-9604. doi: 10.1111/jcmm.15387. Epub 2020 Jul 14. J Cell Mol Med. 2020. PMID: 32666704 Free PMC article.
-
Extracellular vesicles in type 2 diabetes mellitus: key roles in pathogenesis, complications, and therapy.J Extracell Vesicles. 2019 Jun 14;8(1):1625677. doi: 10.1080/20013078.2019.1625677. eCollection 2019. J Extracell Vesicles. 2019. PMID: 31258879 Free PMC article. Review.
-
Exosomes from Endothelial Progenitor Cells Improve the Outcome of a Murine Model of Sepsis.Mol Ther. 2018 May 2;26(5):1375-1384. doi: 10.1016/j.ymthe.2018.02.020. Epub 2018 Feb 27. Mol Ther. 2018. PMID: 29599080 Free PMC article.
-
How to maximize the therapeutic effect of exosomes on skin wounds in diabetes mellitus: Review and discussion.Front Endocrinol (Lausanne). 2023 Mar 27;14:1146991. doi: 10.3389/fendo.2023.1146991. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37051206 Free PMC article. Review.
References
-
- Lioupis C. Effects of diabetes mellitus on wound healing: an update. J Wound Care. 2005;14:84–86. - PubMed
-
- Kant V, Gopal A, Kumar D, Pathak NN, Ram M, Jangir BL, Tandan SK, Kumar D. Curcumin-induced angiogenesis hastens wound healing in diabetic rats. J Surg Res. 2015;193:978–988. - PubMed
-
- Kim JY, Song SH, Kim KL, Ko JJ, Im JE, Yie SW, Ahn YK, Kim DK, Suh W. Human cord blood-derived endothelial progenitor cells and their conditioned media exhibit therapeutic equivalence for diabetic wound healing. Cell Transplant. 2010;19:1635–1644. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

