Gene Therapy With Angiotensin-(1-9) Preserves Left Ventricular Systolic Function After Myocardial Infarction
- PMID: 27978950
- PMCID: PMC5158000
- DOI: 10.1016/j.jacc.2016.09.946
Gene Therapy With Angiotensin-(1-9) Preserves Left Ventricular Systolic Function After Myocardial Infarction
Abstract
Background: Angiotensin-(1-9) [Ang-(1-9)] is a novel peptide of the counter-regulatory axis of the renin-angiotensin-aldosterone system previously demonstrated to have therapeutic potential in hypertensive cardiomyopathy when administered via osmotic mini-pump. Here, we investigate whether gene transfer of Ang-(1-9) is cardioprotective in a murine model of myocardial infarction (MI).
Objectives: The authors evaluated effects of Ang-(1-9) gene therapy on myocardial structural and functional remodeling post-infarction.
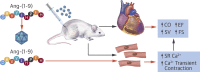
Methods: C57BL/6 mice underwent permanent left anterior descending coronary artery ligation and cardiac function was assessed using echocardiography for 8 weeks followed by a terminal measurement of left ventricular pressure volume loops. Ang-(1-9) was delivered by adeno-associated viral vector via single tail vein injection immediately following induction of MI. Direct effects of Ang-(1-9) on cardiomyocyte excitation/contraction coupling and cardiac contraction were evaluated in isolated mouse and human cardiomyocytes and in an ex vivo Langendorff-perfused whole-heart model.
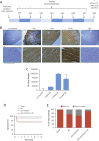
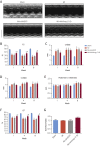
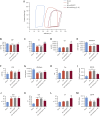
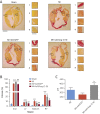
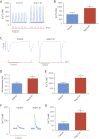
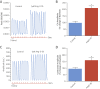
Results: Gene delivery of Ang-(1-9) reduced sudden cardiac death post-MI. Pressure volume measurements revealed complete restoration of end-systolic pressure, ejection fraction, end-systolic volume, and the end-diastolic pressure volume relationship by Ang-(1-9) treatment. Stroke volume and cardiac output were significantly increased versus sham. Histological analysis revealed only mild effects on cardiac hypertrophy and fibrosis, but a significant increase in scar thickness. Direct assessment of Ang-(1-9) on isolated cardiomyocytes demonstrated a positive inotropic effect via increasing calcium transient amplitude and contractility. Ang-(1-9) increased contraction in the Langendorff model through a protein kinase A-dependent mechanism.
Conclusions: Our novel findings showed that Ang-(1-9) gene therapy preserved left ventricular systolic function post-MI, restoring cardiac function. Furthermore, Ang-(1-9) directly affected cardiomyocyte calcium handling through a protein kinase A-dependent mechanism. These data emphasized Ang-(1-9) gene therapy as a potential new strategy in the context of MI.
Keywords: adeno-associated virus; calcium; inotropy; renin angiotensin system.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



Comment in
-
Angiotensin-(1-9): New Promise for Post-Infarct Functional Therapy.J Am Coll Cardiol. 2016 Dec 20;68(24):2667-2669. doi: 10.1016/j.jacc.2016.10.011. J Am Coll Cardiol. 2016. PMID: 27978951 No abstract available.
Similar articles
-
Lentivirus-mediated overexpression of angiotensin-(1-7) attenuated ischaemia-induced cardiac pathophysiology.Exp Physiol. 2011 Sep;96(9):863-74. doi: 10.1113/expphysiol.2011.056994. Epub 2011 Jun 17. Exp Physiol. 2011. PMID: 21685447 Free PMC article.
-
Novel role of aminopeptidase-A in angiotensin-(1-7) metabolism post myocardial infarction.Am J Physiol Heart Circ Physiol. 2014 Apr 1;306(7):H1032-40. doi: 10.1152/ajpheart.00911.2013. Epub 2014 Jan 24. Am J Physiol Heart Circ Physiol. 2014. PMID: 24464749 Free PMC article.
-
Bilateral sympathectomy improves postinfarction left ventricular remodeling and function.J Thorac Cardiovasc Surg. 2017 Apr;153(4):855-863.e1. doi: 10.1016/j.jtcvs.2016.11.037. Epub 2016 Nov 23. J Thorac Cardiovasc Surg. 2017. PMID: 27998611
-
rAAV-mediated angiogenin gene transfer induces angiogenesis and modifies left ventricular remodeling in rats with myocardial infarction.J Mol Med (Berl). 2006 Dec;84(12):1033-46. doi: 10.1007/s00109-006-0092-y. Epub 2006 Sep 6. J Mol Med (Berl). 2006. PMID: 16955274
-
Clinical aspects of left ventricular diastolic function assessed by Doppler echocardiography following acute myocardial infarction.Dan Med Bull. 2001 Nov;48(4):199-210. Dan Med Bull. 2001. PMID: 11767125 Review.
Cited by
-
Large animal models of cardiac ischemia-reperfusion injury: Where are we now?Zool Res. 2023 May 18;44(3):591-603. doi: 10.24272/j.issn.2095-8137.2022.487. Zool Res. 2023. PMID: 37147910 Free PMC article. Review.
-
Communication Between Cardiomyocytes and Fibroblasts During Cardiac Ischemia/Reperfusion and Remodeling: Roles of TGF-β, CTGF, the Renin Angiotensin Axis, and Non-coding RNA Molecules.Front Physiol. 2021 Sep 3;12:716721. doi: 10.3389/fphys.2021.716721. eCollection 2021. Front Physiol. 2021. PMID: 34539441 Free PMC article. Review.
-
Angiotensin-(1-9) attenuates adriamycin-induced cardiomyopathy in rats via the angiotensin type 2 receptor.Mol Cell Biochem. 2024 Jan;479(1):73-83. doi: 10.1007/s11010-023-04718-y. Epub 2023 Mar 30. Mol Cell Biochem. 2024. PMID: 36995547
-
Biologics and their delivery systems: Trends in myocardial infarction.Adv Drug Deliv Rev. 2021 Jun;173:181-215. doi: 10.1016/j.addr.2021.03.014. Epub 2021 Mar 26. Adv Drug Deliv Rev. 2021. PMID: 33775706 Free PMC article. Review.
-
Counter-regulatory renin-angiotensin system in cardiovascular disease.Nat Rev Cardiol. 2020 Feb;17(2):116-129. doi: 10.1038/s41569-019-0244-8. Epub 2019 Aug 19. Nat Rev Cardiol. 2020. PMID: 31427727 Free PMC article. Review.
References
-
- Donoghue M., Hsieh F., Baronas E. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1–E9. - PubMed
-
- Tipnis S.R., Hooper N.M., Hyde R., Karran E., Christie G., Turner A.J. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. - PubMed
-
- Der Sarkissian S., Huentelman M.J., Stewart J., Katovich M.J., Raizada M.K. ACE2: a novel therapeutic target for cardiovascular diseases. Prog Biophys Mol Biol. 2006;91:163–198. - PubMed
-
- Ferreira A.J., Santos R.A., Almeida A.P. Angiotensin-(1-7): cardioprotective effect in myocardial ischemia/reperfusion. Hypertension. 2001;38:665–668. - PubMed
-
- Grobe J.L., Mecca A.P., Mao H., Katovich M.J. Chronic angiotensin-(1-7) prevents cardiac fibrosis in DOCA-salt model of hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H2417–H2423. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

