Arteriogenesis in murine adipose tissue is contingent on CD68+ /CD206+ macrophages
- PMID: 27976451
- PMCID: PMC5432396
- DOI: 10.1111/micc.12341
Arteriogenesis in murine adipose tissue is contingent on CD68+ /CD206+ macrophages
Abstract
Objective: The surgical transfer of skin, fat, and/or muscle from a donor site to a recipient site within the same patient is a widely performed procedure in reconstructive surgeries. A surgical pretreatment strategy that is intended to increase perfusion in the flap, termed "flap delay," is a commonly employed technique by plastic surgeons prior to flap transplantation. Here, we explored whether CD68+ /CD206+ macrophages are required for arteriogenesis within the flap by performing gain-of-function and loss-of-function studies in a previously published flap delay murine model.
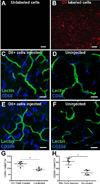
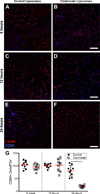
Methods and results: Local injection of M2-polarized macrophages into the flap resulted in an increase in collateral vessel diameter. Application of a thin biomaterial film loaded with a pharmacological agent (FTY720), which has been previously shown to recruit CD68+ /CD206+ macrophages to remodeling tissue, increased CD68+ /CD206+ cell recruitment and collateral vessel enlargement. Conversely, when local macrophage populations were depleted within the inguinal fat pad via clodronate liposome delivery, we observed fewer CD68+ cells accompanied by diminished collateral vessel enlargement.
Conclusions: Our study underscores the importance of macrophages during microvascular adaptations that are induced by flap delay. These studies suggest a mechanism for a translatable therapeutic target that may be used to enhance the clinical flap delay procedure.
Keywords: adipose; arteriogenesis; collateral vessel; macrophage.
© 2016 John Wiley & Sons Ltd.
Figures




Similar articles
-
Monocytes are recruited from venules during arteriogenesis in the murine spinotrapezius ligation model.Arterioscler Thromb Vasc Biol. 2014 Sep;34(9):2012-22. doi: 10.1161/ATVBAHA.114.303399. Epub 2014 Jun 26. Arterioscler Thromb Vasc Biol. 2014. PMID: 24969773 Free PMC article.
-
Macrophage Recruitment and Polarization During Collateral Vessel Remodeling in Murine Adipose Tissue.Microcirculation. 2016 Jan;23(1):75-87. doi: 10.1111/micc.12261. Microcirculation. 2016. PMID: 26638986 Free PMC article.
-
Human adipose tissue macrophages: m1 and m2 cell surface markers in subcutaneous and omental depots and after weight loss.J Clin Endocrinol Metab. 2009 Nov;94(11):4619-23. doi: 10.1210/jc.2009-0925. Epub 2009 Oct 16. J Clin Endocrinol Metab. 2009. PMID: 19837929
-
Antibody combinations for optimized staining of macrophages in human lung tumours.Scand J Immunol. 2020 Jul;92(1):e12889. doi: 10.1111/sji.12889. Epub 2020 May 10. Scand J Immunol. 2020. PMID: 32299134
-
Significance of M2 macrophages in glomerulonephritis with crescents.Pathol Res Pract. 2017 Sep;213(9):1215-1220. doi: 10.1016/j.prp.2017.04.011. Epub 2017 Apr 20. Pathol Res Pract. 2017. PMID: 28554749
Cited by
-
Multiplatform Single-Cell Analysis Identifies Immune Cell Types Enhanced in Pulmonary Fibrosis.Am J Respir Cell Mol Biol. 2022 Jul;67(1):50-60. doi: 10.1165/rcmb.2021-0418OC. Am J Respir Cell Mol Biol. 2022. PMID: 35468042 Free PMC article.
-
Cellular and Molecular Heterogeneity Associated with Vessel Formation Processes.Biomed Res Int. 2018 Oct 10;2018:6740408. doi: 10.1155/2018/6740408. eCollection 2018. Biomed Res Int. 2018. PMID: 30406137 Free PMC article. Review.
-
The Immunosuppressant Fingolimod (FTY720) for the Treatment of Mechanical Force-Induced Abnormal Scars.J Immunol Res. 2020 Jan 7;2020:7057195. doi: 10.1155/2020/7057195. eCollection 2020. J Immunol Res. 2020. PMID: 32377536 Free PMC article.
References
-
- Awojoodu AO, Ogle ME, Sefcik LS, Bowers DT, Martin K, Brayman KL, Lynch KR, Peirce-Cottler SM, Botchwey E. Sphingosine 1-phosphate receptor 3 regulates recruitment of anti-inflammatory monocytes to microvessels during implant arteriogenesis. Proc. Natl. Acad. Sci. U. S. A. 2013;110:13785–13790. - PMC - PubMed
-
- Biewenga J, van der Ende MB, Krist LF, Borst A, Ghufron M, van Rooijen N. Macrophage depletion in the rat after intraperitoneal administration of liposome-encapsulated clodronate: depletion kinetics and accelerated repopulation of peritoneal and omental macrophages by administration of Freund’s adjuvant. Cell Tissue Res. 1995;280:189–196. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

