Protein S-Bacillithiolation Functions in Thiol Protection and Redox Regulation of the Glyceraldehyde-3-Phosphate Dehydrogenase Gap in Staphylococcus aureus Under Hypochlorite Stress
- PMID: 27967218
- PMCID: PMC5791933
- DOI: 10.1089/ars.2016.6897
Protein S-Bacillithiolation Functions in Thiol Protection and Redox Regulation of the Glyceraldehyde-3-Phosphate Dehydrogenase Gap in Staphylococcus aureus Under Hypochlorite Stress
Abstract
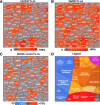
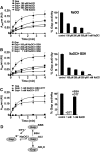
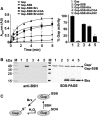
Aims: Bacillithiol (BSH) is the major low-molecular-weight thiol of the human pathogen Staphylococcus aureus. In this study, we used OxICAT and Voronoi redox treemaps to quantify hypochlorite-sensitive protein thiols in S. aureus USA300 and analyzed the role of BSH in protein S-bacillithiolation.
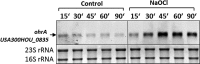
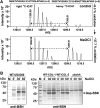
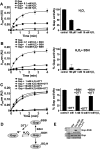
Results: The OxICAT analyses enabled the quantification of 228 Cys residues in the redox proteome of S. aureus USA300. Hypochlorite stress resulted in >10% increased oxidation of 58 Cys residues (25.4%) in the thiol redox proteome. Among the highly oxidized sodium hypochlorite (NaOCl)-sensitive proteins are five S-bacillithiolated proteins (Gap, AldA, GuaB, RpmJ, and PpaC). The glyceraldehyde-3-phosphate (G3P) dehydrogenase Gap represents the most abundant S-bacillithiolated protein contributing 4% to the total Cys proteome. The active site Cys151 of Gap was very sensitive to overoxidation and irreversible inactivation by hydrogen peroxide (H2O2) or NaOCl in vitro. Treatment with H2O2 or NaOCl in the presence of BSH resulted in reversible Gap inactivation due to S-bacillithiolation, which could be regenerated by the bacilliredoxin Brx (SAUSA300_1321) in vitro. Molecular docking was used to model the S-bacillithiolated Gap active site, suggesting that formation of the BSH mixed disulfide does not require major structural changes. Conclusion and Innovation: Using OxICAT analyses, we identified 58 novel NaOCl-sensitive proteins in the pathogen S. aureus that could play protective roles against the host immune defense and include the glycolytic Gap as major target for S-bacillithiolation. S-bacillithiolation of Gap did not require structural changes, but efficiently functions in redox regulation and protection of the active site against irreversible overoxidation in S. aureus. Antioxid. Redox Signal. 28, 410-430.
Keywords: Gap; S-bacillithiolation; Staphylococcus aureus; bacilliredoxin; thiol-redox proteomics.
Conflict of interest statement
No competing financial interests exist.
Figures



Similar articles
-
The aldehyde dehydrogenase AldA contributes to the hypochlorite defense and is redox-controlled by protein S-bacillithiolation in Staphylococcus aureus.Redox Biol. 2018 May;15:557-568. doi: 10.1016/j.redox.2018.02.001. Epub 2018 Feb 5. Redox Biol. 2018. PMID: 29433022 Free PMC article.
-
Real-Time Imaging of the Bacillithiol Redox Potential in the Human Pathogen Staphylococcus aureus Using a Genetically Encoded Bacilliredoxin-Fused Redox Biosensor.Antioxid Redox Signal. 2017 May 20;26(15):835-848. doi: 10.1089/ars.2016.6733. Epub 2016 Aug 11. Antioxid Redox Signal. 2017. PMID: 27462976 Free PMC article.
-
S-bacillithiolation protects against hypochlorite stress in Bacillus subtilis as revealed by transcriptomics and redox proteomics.Mol Cell Proteomics. 2011 Nov;10(11):M111.009506. doi: 10.1074/mcp.M111.009506. Epub 2011 Jul 11. Mol Cell Proteomics. 2011. PMID: 21749987 Free PMC article.
-
The Role of Bacillithiol in Gram-Positive Firmicutes.Antioxid Redox Signal. 2018 Feb 20;28(6):445-462. doi: 10.1089/ars.2017.7057. Epub 2017 Apr 24. Antioxid Redox Signal. 2018. PMID: 28301954 Free PMC article. Review.
-
Thiol-based redox switches in the major pathogen Staphylococcus aureus.Biol Chem. 2020 Nov 23;402(3):333-361. doi: 10.1515/hsz-2020-0272. Print 2021 Feb 23. Biol Chem. 2020. PMID: 33544504 Review.
Cited by
-
Profiling the Site of Protein CoAlation and Coenzyme A Stabilization Interactions.Antioxidants (Basel). 2022 Jul 14;11(7):1362. doi: 10.3390/antiox11071362. Antioxidants (Basel). 2022. PMID: 35883853 Free PMC article.
-
Protein CoAlation and antioxidant function of coenzyme A in prokaryotic cells.Biochem J. 2018 Jun 6;475(11):1909-1937. doi: 10.1042/BCJ20180043. Biochem J. 2018. PMID: 29626155 Free PMC article.
-
Sulfinamide Formation from the Reaction of Bacillithiol and Nitroxyl.ACS Chem Biol. 2023 Dec 15;18(12):2524-2534. doi: 10.1021/acschembio.3c00526. Epub 2023 Nov 27. ACS Chem Biol. 2023. PMID: 38012810 Free PMC article.
-
Redefining the Clostridioides difficile σB Regulon: σB Activates Genes Involved in Detoxifying Radicals That Can Result from the Exposure to Antimicrobials and Hydrogen Peroxide.mSphere. 2020 Sep 16;5(5):e00728-20. doi: 10.1128/mSphere.00728-20. mSphere. 2020. PMID: 32938698 Free PMC article.
-
The Catalase KatA Contributes to Microaerophilic H2O2 Priming to Acquire an Improved Oxidative Stress Resistance in Staphylococcus aureus.Antioxidants (Basel). 2022 Sep 12;11(9):1793. doi: 10.3390/antiox11091793. Antioxidants (Basel). 2022. PMID: 36139867 Free PMC article.
References
-
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, and Zwart PH. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66: 213–221, 2010 - PMC - PubMed
-
- Archer GL. Staphylococcus aureus: a well-armed pathogen. Clin Infect Dis 26: 1179–1181, 1998 - PubMed
-
- Bedhomme M, Adamo M, Marchand CH, Couturier J, Rouhier N, Lemaire SD, Zaffagnini M, and Trost P. Glutathionylation of cytosolic glyceraldehyde-3-phosphate dehydrogenase from the model plant Arabidopsis thaliana is reversed by both glutaredoxins and thioredoxins in vitro. Biochem J 445: 337–347, 2012 - PubMed
-
- Blanc B, Gerez C, and Ollagnier de Choudens S. Assembly of Fe/S proteins in bacterial systems: biochemistry of the bacterial ISC system. Biochim Biophys Acta 1853: 1436–1447, 2015 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

