Attenuation of chemokine receptor function and surface expression as an immunomodulatory strategy employed by human cytomegalovirus is linked to vGPCR US28
- PMID: 27955674
- PMCID: PMC5153698
- DOI: 10.1186/s12964-016-0154-x
Attenuation of chemokine receptor function and surface expression as an immunomodulatory strategy employed by human cytomegalovirus is linked to vGPCR US28
Abstract
Background: Some herpesviruses like human cytomegalovirus (HCMV) encode viral G protein-coupled receptors that cause reprogramming of cell signaling to facilitate dissemination of the virus, prevent immune surveillance and establish life-long latency. Human GPCRs are known to function in complex signaling networks involving direct physical interactions as well as indirect crosstalk of orthogonal signaling networks. The human chemokine receptor CXCR4 is expressed on hematopoietic stem cells, leukocytes, endothelial and epithelial cells, which are infected by HCMV or display reservoirs of latency.
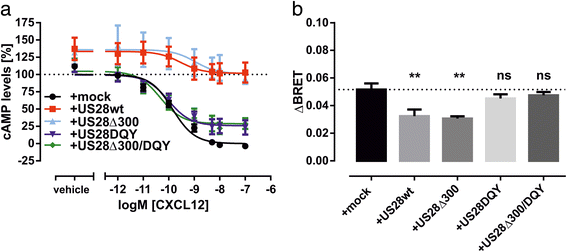
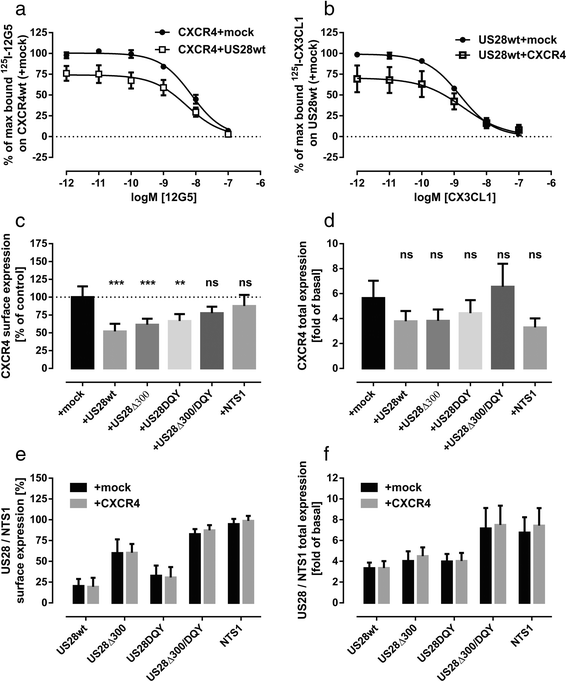
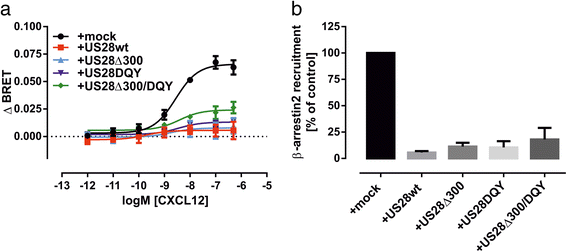
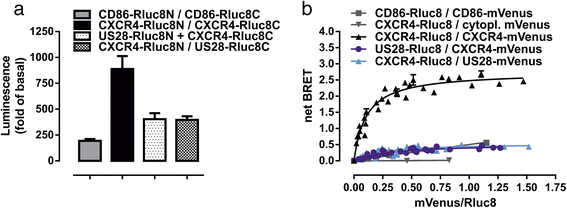
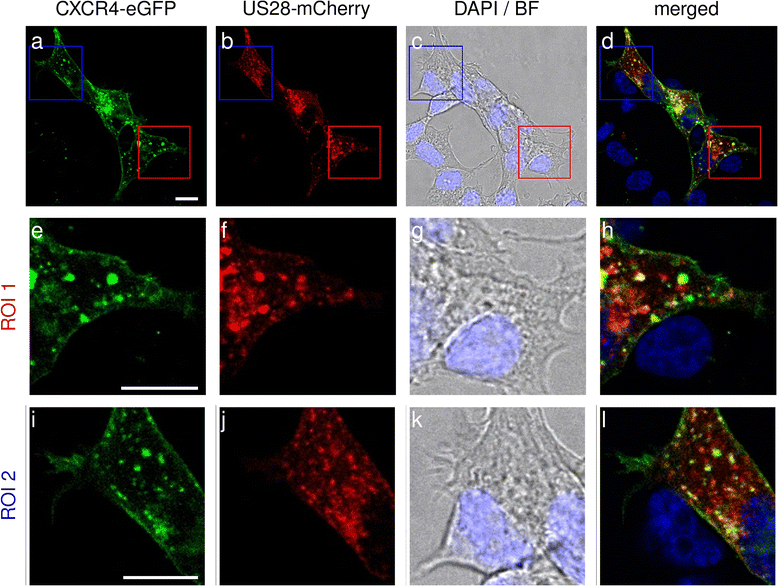
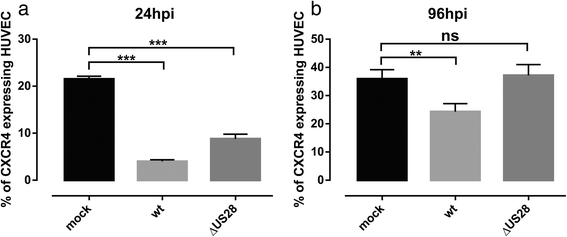
Results: We investigated the potential heteromerization of US28 with CXCR4 as well as the influence of US28 on CXCR4 signaling. Using Bioluminescence Resonance Energy Transfer and luciferase-complementation based methods we show that US28 expression exhibits negative effects on CXCR4 signaling and constitutive surface expression in HEK293T cells. Furthermore, we demonstrate that this effect is not mediated by receptor heteromerization but via signaling crosstalk. Additionally, we show that in HCMV, strain TB40E, infected HUVEC the surface expression of CXCR4 is strongly downregulated, whereas in TB40E-delUS28 infected cells, CXCR4 surface expression is not altered in particular at late time points of infection.
Conclusions: We show that the vGPCR US28 is leading to severely disturbed signaling and surface expression of the chemokine receptor CXCR4 thereby representing an effective mechanism used by vGPCRs to reprogram host cell signaling. In contrast to other studies, we demonstrate that these effects are not mediated via heteromerization.
Keywords: Bioluminescence complementation; Bioluminescence resonance energy transfer; Chemokine receptor CXCR4; Constitutive activity; Signaling crosstalk; Viral G protein-coupled receptor US28.
Figures
Similar articles
-
The Human Cytomegalovirus US27 Gene Product Constitutively Activates Antioxidant Response Element-Mediated Transcription through Gβγ, Phosphoinositide 3-Kinase, and Nuclear Respiratory Factor 1.J Virol. 2018 Nov 12;92(23):e00644-18. doi: 10.1128/JVI.00644-18. Print 2018 Dec 1. J Virol. 2018. PMID: 30209167 Free PMC article.
-
Human Cytomegalovirus UL111A and US27 Gene Products Enhance the CXCL12/CXCR4 Signaling Axis via Distinct Mechanisms.J Virol. 2018 Feb 12;92(5):e01981-17. doi: 10.1128/JVI.01981-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29237840 Free PMC article.
-
Human Cytomegalovirus-Encoded Receptor US28 Is Expressed in Renal Allografts and Facilitates Viral Spreading In Vitro.Transplantation. 2017 Mar;101(3):531-540. doi: 10.1097/TP.0000000000001289. Transplantation. 2017. PMID: 27362315
-
HCMV-encoded G-protein-coupled receptors as constitutively active modulators of cellular signaling networks.Trends Pharmacol Sci. 2006 Jan;27(1):56-63. doi: 10.1016/j.tips.2005.11.006. Epub 2005 Dec 13. Trends Pharmacol Sci. 2006. PMID: 16352349 Review.
-
US28 actions in HCMV infection: lessons from a versatile hijacker.Rev Med Virol. 2005 Jul-Aug;15(4):269-82. doi: 10.1002/rmv.468. Rev Med Virol. 2005. PMID: 15861487 Review.
Cited by
-
Viral G Protein-Coupled Receptors Encoded by β- and γ-Herpesviruses.Annu Rev Virol. 2022 Sep 29;9(1):329-351. doi: 10.1146/annurev-virology-100220-113942. Epub 2022 Jun 7. Annu Rev Virol. 2022. PMID: 35671566 Free PMC article. Review.
-
Atypical structural snapshots of human cytomegalovirus GPCR interactions with host G proteins.Sci Adv. 2022 Jan 21;8(3):eabl5442. doi: 10.1126/sciadv.abl5442. Epub 2022 Jan 21. Sci Adv. 2022. PMID: 35061538 Free PMC article.
-
Identification of a novel signaling complex containing host chemokine receptor CXCR4, Interleukin-10 receptor, and human cytomegalovirus US27.Virology. 2020 Sep;548:49-58. doi: 10.1016/j.virol.2020.06.006. Epub 2020 Jun 17. Virology. 2020. PMID: 32838946 Free PMC article.
-
The Human Cytomegalovirus US27 Gene Product Constitutively Activates Antioxidant Response Element-Mediated Transcription through Gβγ, Phosphoinositide 3-Kinase, and Nuclear Respiratory Factor 1.J Virol. 2018 Nov 12;92(23):e00644-18. doi: 10.1128/JVI.00644-18. Print 2018 Dec 1. J Virol. 2018. PMID: 30209167 Free PMC article.
-
US28: HCMV's Swiss Army Knife.Viruses. 2018 Aug 20;10(8):445. doi: 10.3390/v10080445. Viruses. 2018. PMID: 30127279 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

