Effects of LRRK2 Inhibitors on Nigrostriatal Dopaminergic Neurotransmission
- PMID: 27943591
- PMCID: PMC5248597
- DOI: 10.1111/cns.12660
Effects of LRRK2 Inhibitors on Nigrostriatal Dopaminergic Neurotransmission
Abstract
Introduction: Mutations in leucine-rich repeat kinase 2 (LRRK2) are the most prevalent cause of familial and sporadic Parkinson's disease (PD). Because most pathogenic LRRK2 mutations result in enhanced kinase activity, it suggests that LRRK2 inhibitors may serve as a potential treatment for PD. To evaluate whether LRRK2 inhibitors are effective therapies for PD, it is crucial to know whether LRRK2 inhibitors will affect dopaminergic (DAergic) neurotransmission. However, to date, there is no study to investigate the impact of LRRK2 inhibitors on DAergic neurotransmission.
Aims: To address this gap in knowledge, we examined the effects of three types of LRRK2 inhibitors (LRRK2-IN-1, GSK2578215A, and GNE-7915) on dopamine (DA) release in the dorsal striatum using fast-scan cyclic voltammetry and DA neuron firing in the substantia nigra pars compacta (SNpc) using patch clamp in mouse brain slices.
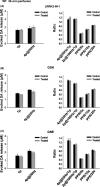
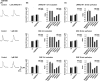
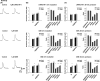
Results: We found that LRRK2-IN-1 at a concentration higher than 1 μM causes off-target effects and decreases DA release, whereas GSK2578215A and GNE-7915 do not. All three inhibitors at 1 μM have no effect on DA release and DA neuron firing rate. We have further assessed the effects of the inhibitors in two preclinical LRRK2 mouse models (i.e., BAC transgenic hG2019S and hR1441G) and demonstrated that GNE-7915 enhances DA release and synaptic vesicle mobilization/recycling.
Conclusion: GNE-7915 can be validated for further therapeutic development for PD.
Keywords: Dopamine; Fast-scan cyclic voltammetry; Inhibitor; LRRK2; Parkinson's disease.
© 2016 The Authors. CNS Neuroscience & Therapeutics Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
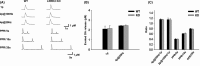


Similar articles
-
Leucine-rich repeat kinase 2 (LRRK2) inhibitors differentially modulate glutamate release and Serine935 LRRK2 phosphorylation in striatal and cerebrocortical synaptosomes.Pharmacol Res Perspect. 2019 May 27;7(3):e00484. doi: 10.1002/prp2.484. eCollection 2019 Jun. Pharmacol Res Perspect. 2019. PMID: 31149340 Free PMC article.
-
LRRK2 BAC transgenic rats develop progressive, L-DOPA-responsive motor impairment, and deficits in dopamine circuit function.Hum Mol Genet. 2016 Mar 1;25(5):951-63. doi: 10.1093/hmg/ddv628. Epub 2016 Jan 6. Hum Mol Genet. 2016. PMID: 26744332 Free PMC article.
-
(G2019S) LRRK2 causes early-phase dysfunction of SNpc dopaminergic neurons and impairment of corticostriatal long-term depression in the PD transgenic mouse.Neurobiol Dis. 2014 Aug;68:190-9. doi: 10.1016/j.nbd.2014.04.021. Epub 2014 May 14. Neurobiol Dis. 2014. PMID: 24830390
-
LRRK2 mouse models: dissecting the behavior, striatal neurochemistry and neurophysiology of PD pathogenesis.Biochem Soc Trans. 2017 Feb 8;45(1):113-122. doi: 10.1042/BST20160238. Biochem Soc Trans. 2017. PMID: 28202664 Review.
-
LRRK2 and Parkinson's Disease: From Lack of Structure to Gain of Function.Curr Protein Pept Sci. 2017;18(7):677-686. doi: 10.2174/1389203717666160311121748. Curr Protein Pept Sci. 2017. PMID: 26965688 Review.
Cited by
-
Recent Developments in LRRK2-Targeted Therapy for Parkinson's Disease.Drugs. 2019 Jul;79(10):1037-1051. doi: 10.1007/s40265-019-01139-4. Drugs. 2019. PMID: 31161537 Review.
-
Personalized medicine in Parkinson's disease: Time to be precise.Mov Disord. 2017 Aug;32(8):1147-1154. doi: 10.1002/mds.27027. Epub 2017 Jun 12. Mov Disord. 2017. PMID: 28605054 Free PMC article. No abstract available.
-
LRRK2-G2019S mice display alterations in glutamatergic synaptic transmission in midbrain dopamine neurons.J Neurochem. 2022 Apr;161(2):158-172. doi: 10.1111/jnc.15588. Epub 2022 Feb 27. J Neurochem. 2022. PMID: 35152441 Free PMC article.
-
The Role of Rab Proteins in Parkinson's Disease Synaptopathy.Biomedicines. 2022 Aug 10;10(8):1941. doi: 10.3390/biomedicines10081941. Biomedicines. 2022. PMID: 36009486 Free PMC article. Review.
-
Aberrant somatic calcium channel function in cNurr1 and LRRK2-G2019S mice.NPJ Parkinsons Dis. 2023 Apr 7;9(1):56. doi: 10.1038/s41531-023-00500-5. NPJ Parkinsons Dis. 2023. PMID: 37029193 Free PMC article.
References
-
- Lees AJ, Hardy J, Revesz T. Parkinson's disease. Lancet 2009;373:2055–2066. - PubMed
-
- Zimprich A, Biskup S, Leitner P, et al. Mutations in LRRK2 cause autosomal‐dominant parkinsonism with pleomorphic pathology. Neuron 2004;44:601–607. - PubMed
-
- Gilks WP, Abou‐Sleiman PM, Gandhi S, et al. A common LRRK2 mutation in idiopathic Parkinson's disease. Lancet 2005;365:415–416. - PubMed
-
- Paisan‐Ruiz C, Jain S, Evans EW, et al. Cloning of the gene containing mutations that cause PARK8‐linked Parkinson's disease. Neuron 2004;44:595–600. - PubMed
-
- Di Fonzo A, Rohe CF, Ferreira J, et al. A frequent LRRK2 gene mutation associated with autosomal dominant Parkinson's disease. Lancet 2005;365:412–415. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

