Efavirenz or nevirapine in three-drug combination therapy with two nucleoside or nucleotide-reverse transcriptase inhibitors for initial treatment of HIV infection in antiretroviral-naïve individuals
- PMID: 27943261
- PMCID: PMC5450880
- DOI: 10.1002/14651858.CD004246.pub4
Efavirenz or nevirapine in three-drug combination therapy with two nucleoside or nucleotide-reverse transcriptase inhibitors for initial treatment of HIV infection in antiretroviral-naïve individuals
Abstract
Background: The advent of highly active antiretroviral therapy (ART) has reduced the morbidity and mortality due to HIV infection. The World Health Organization (WHO) ART guidelines focus on three classes of antiretroviral drugs, namely nucleoside or nucleotide reverse transcriptase inhibitors (NRTI), non-nucleoside reverse transcriptase inhibitors (NNRTI) and protease inhibitors. Two of the most common medications given as first-line treatment are the NNRTIs, efavirenz (EFV) and nevirapine (NVP). It is unclear which NNRTI is more efficacious for initial therapy. This systematic review was first published in 2010.
Objectives: To determine which non-nucleoside reverse transcriptase inhibitor, either EFV or NVP, is more effective in suppressing viral load when given in combination with two nucleoside reverse transcriptase inhibitors as part of initial antiretroviral therapy for HIV infection in adults and children.
Search methods: We attempted to identify all relevant studies, regardless of language or publication status, in electronic databases and conference proceedings up to 12 August 2016. We searched MEDLINE, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL), the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) and ClinicalTrials.gov to 12 August 2016. We searched LILACS (Latin American and Caribbean Health Sciences Literature) and the Web of Science from 1996 to 12 August 2016. We checked the National Library of Medicine (NLM) Gateway from 1996 to 2009, as it was no longer available after 2009.
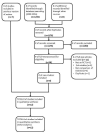
Selection criteria: We included all randomized controlled trials (RCTs) that compared EFV to NVP in people with HIV without prior exposure to ART, irrespective of the dosage or NRTI's given in combination.The primary outcome of interest was virological success. Other primary outcomes included mortality, clinical progression to AIDS, severe adverse events, and discontinuation of therapy for any reason. Secondary outcomes were change in CD4 count, treatment failure, development of ART drug resistance, and prevention of sexual transmission of HIV.
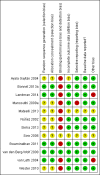
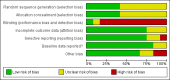
Data collection and analysis: Two review authors assessed each reference for inclusion using exclusion criteria that we had established a priori. Two review authors independently extracted data from each included trial using a standardized data extraction form. We analysed data on an intention-to-treat basis. We performed subgroup analyses for concurrent treatment for tuberculosis and dosage of NVP. We followed standard Cochrane methodological procedures.
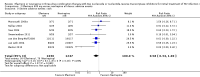
Main results: Twelve RCTs, which included 3278 participants, met our inclusion criteria. None of these trials included children. The length of follow-up time, study settings, and NRTI combination drugs varied greatly. In five included trials, participants were receiving concurrent treatment for tuberculosis.There was little or no difference between EFV and NVP in virological success (RR 1.04, 95% CI 0.99 to 1.09; 10 trials, 2438 participants; high quality evidence), probably little or no difference in mortality (RR 0.84, 95% CI 0.59 to 1.19; 8 trials, 2317 participants; moderate quality evidence) and progression to AIDS (RR 1.23, 95% CI 0.72 to 2.11; 5 trials, 2005 participants; moderate quality evidence). We are uncertain whether there is a difference in all severe adverse events (RR 0.91, 95% CI 0.71 to 1.18; 8 trials, 2329 participants; very low quality evidence). There is probably little or no difference in discontinuation rate (RR 0.93, 95% CI 0.69 to 1.25; 9 trials, 2384 participants; moderate quality evidence) and change in CD4 count (MD -3.03; 95% CI -17.41 to 11.35; 9 trials, 1829 participants; moderate quality evidence). There may be little or no difference in treatment failure (RR 0.97, 95% CI 0.76 to 1.24; 5 trials, 737 participants; low quality evidence). Development of drug resistance is probably slightly less in the EFV arms (RR 0.76, 95% CI 0.60 to 0.95; 4 trials, 988 participants; moderate quality evidence). No studies were found that looked at sexual transmission of HIV.When we examined the adverse events individually, EFV probably is associated with more people with impaired mental function (7 per 1000) compared to NVP (2 per 1000; RR 4.46, 95% CI 1.65 to 12.03; 6 trials, 2049 participants; moderate quality evidence) but fewer people with elevated transaminases (RR 0.52, 95% CI 0.35 to 0.78; 3 trials, 1299 participants; high quality evidence), fewer people with neutropenia (RR 0.48, 95% CI 0.28 to 0.82; 3 trials, 1799 participants; high quality evidence), and probably fewer people withrash (229 per 100 with NVP versus 133 per 1000 with EFV; RR 0.58, 95% CI 0.34 to 1.00; 7 trials, 2277 participants; moderate quality evidence). We found that there may be little or no difference in gastrointestinal adverse events (RR 0.76, 95% CI 0.48 to 1.21; 6 trials, 2049 participants; low quality evidence), pyrexia (RR 0.65, 95% CI 0.15 to 2.73; 3 trials, 1799 participants; low quality evidence), raised alkaline phosphatase (RR 0.65, 95% CI 0.17 to 2.50; 1 trial, 1007 participants; low quality evidence), raised amylase (RR 1.40, 95% CI 0.72 to 2.73; 2 trials, 1071 participants; low quality evidence) and raised triglycerides (RR 1.10, 95% CI 0.39 to 3.13; 2 trials, 1071 participants; low quality evidence). There was probably little or no difference in serum glutamic oxaloacetic transaminase (SGOT; MD 3.3, 95% CI -2.06 to 8.66; 1 trial, 135 participants; moderate quality evidence), serum glutamic- pyruvic transaminase (SGPT; MD 5.7, 95% CI -4.23 to 15.63; 1 trial, 135 participants; moderate quality evidence) and raised cholesterol (RR 6.03, 95% CI 0.75 to 48.78; 1 trial, 64 participants; moderate quality evidence).Our subgroup analyses revealed that NVP slightly increases mortality when given once daily (RR 0.34, 95% CI 0.13 to 0.90; 3 trials, 678 participants; high quality evidence). There were little or no differences in the primary outcomes for patients who were concurrently receiving treatment for tuberculosis.
Authors' conclusions: Both drugs have similar benefits in initial treatment of HIV infection when combined with two NRTIs. The adverse events encountered affect different systems, with EFV more likely to cause central nervous system adverse events and NVP more likely to raise transaminases, cause neutropenia and rash.
Conflict of interest statement
Disclaimer: we prepared this article while Alicen Spaulding was employed at the University of Minnesota. The opinions expressed in this article are those of the review authors' and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States Government. Alice Spaulding received salary support from the WHO for this project. Lawrence Mbuagbaw, James Irlam, Sara Mursleen and George Rutherford have no known conflicts of interest. Nandi Siegfried provides technical consultation on the efficacy of drugs for a managed care organization (MEDSCHEME), for which she receives an honorarium.
Figures



























Update of
-
Efavirenz or nevirapine in three-drug combination therapy with two nucleoside-reverse transcriptase inhibitors for initial treatment of HIV infection in antiretroviral-naïve individuals.Cochrane Database Syst Rev. 2010 Dec 8;(12):CD004246. doi: 10.1002/14651858.CD004246.pub3. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2016 Dec 10;12:CD004246. doi: 10.1002/14651858.CD004246.pub4. PMID: 21154355 Updated. Review.
Similar articles
-
Efavirenz or nevirapine in three-drug combination therapy with two nucleoside-reverse transcriptase inhibitors for initial treatment of HIV infection in antiretroviral-naïve individuals.Cochrane Database Syst Rev. 2010 Dec 8;(12):CD004246. doi: 10.1002/14651858.CD004246.pub3. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2016 Dec 10;12:CD004246. doi: 10.1002/14651858.CD004246.pub4. PMID: 21154355 Updated. Review.
-
Tenofovir or zidovudine in three-drug combination therapy with one nucleoside reverse transcriptase inhibitor and one non-nucleoside reverse transcriptase inhibitor for initial treatment of HIV infection in antiretroviral-naïve individuals.Cochrane Database Syst Rev. 2010 Oct 6;(10):CD008740. doi: 10.1002/14651858.CD008740. Cochrane Database Syst Rev. 2010. PMID: 20927777 Review.
-
Abacavir-based triple nucleoside regimens for maintenance therapy in patients with HIV.Cochrane Database Syst Rev. 2013 Jun 5;2013(6):CD008270. doi: 10.1002/14651858.CD008270.pub2. Cochrane Database Syst Rev. 2013. PMID: 23740608 Free PMC article. Review.
-
Optimisation of antiretroviral therapy in HIV-infected children under 3 years of age.Cochrane Database Syst Rev. 2014 May 22;2014(5):CD004772. doi: 10.1002/14651858.CD004772.pub4. Cochrane Database Syst Rev. 2014. PMID: 24852077 Free PMC article. Review.
-
Antiretroviral resistance testing in HIV-positive people.Cochrane Database Syst Rev. 2018 Nov 9;11(11):CD006495. doi: 10.1002/14651858.CD006495.pub5. Cochrane Database Syst Rev. 2018. PMID: 30411789 Free PMC article.
Cited by
-
A retrospective observation of virologically suppressed people living with HIV by comparing switching to BIC/TAF/FTC with initial use BIC/TAF/FTC.Ann Med. 2023;55(2):2305692. doi: 10.1080/07853890.2024.2305692. Epub 2024 Jan 18. Ann Med. 2023. PMID: 38237196 Free PMC article.
-
Population-Specific Predictors of Immunologic Reconstitution Following Initiation of Combined Antiretroviral Therapy in Children: A Retrospective Observational Study from a 15-Year Cohort of HIV-Positive Children and Adolescents in Eritrea.HIV AIDS (Auckl). 2024 Nov 6;16:433-453. doi: 10.2147/HIV.S483094. eCollection 2024. HIV AIDS (Auckl). 2024. PMID: 39524019 Free PMC article.
-
Conjunctival Flora of Human Immunodeficiency Virus Patients on Antiretroviral Treatment.Ophthalmol Eye Dis. 2017 Aug 3;9:1179172117724760. doi: 10.1177/1179172117724760. eCollection 2017. Ophthalmol Eye Dis. 2017. PMID: 28814905 Free PMC article.
-
Acute Liver Failure among Patients on Efavirenz-Based Antiretroviral Therapy.Case Reports Hepatol. 2018 May 10;2018:1270716. doi: 10.1155/2018/1270716. eCollection 2018. Case Reports Hepatol. 2018. PMID: 29862098 Free PMC article.
-
Pre-treatment HIV-drug resistance associated with virologic outcome of first-line NNRTI-antiretroviral therapy: A cohort study in Kenya.EClinicalMedicine. 2020 Jan 14;18:100239. doi: 10.1016/j.eclinm.2019.100239. eCollection 2020 Jan. EClinicalMedicine. 2020. PMID: 31956856 Free PMC article.
References
References to studies included in this review
Ayala Gaytán 2004 {published data only}
-
- Ayala Gaytán JJ, Zapata de La Garza ER, Chávez García M, Valdovinos Chávez SB. [Nevirapine or efavirenz in combination with two nucleoside analogues in HIV‐infected antiretroviral‐naïve patients]. Medicina Interna de México 2004;20(1):24‐33. [CN‐00641209]
Bonnet 2013a {published data only}
-
- Bonnet M, Bhatt N, Baudin E, Silva C, Michon C, Taburet AM, et al. Nevirapine versus efavirenz for patients co‐infected with HIV and tuberculosis: a randomised non‐inferiority trial. Lancet Infectious diseases 2013;13(4):303‐12. [PUBMED: 23433590] - PubMed
Landman 2014 {published data only}
-
- Landman R, Koulla‐Shiro S, Sow PS, Ngolle M, Diallo MB, Guèye NF, et al. Evaluation of four tenofovir‐containing regimens as first‐line treatments in Cameroon and Senegal: the ANRS 12115 DAYANA Trial. Antiviral Therapy 2014;19(1):51‐9. [PUBMED: 23970206] - PubMed
Manosuthi 2009a {published data only}
-
- Manosuthi W, Sungkanuparph S, Tantanathip P, Lueangniyomkul A, Mankatitham W, Prasithsirskul W, et al. A randomized trial comparing plasma drug concentrations and efficacies between 2 nonnucleoside reverse‐transcriptase inhibitor‐based regimens in HIV‐infected patients receiving rifampicin: the N2R Study. Clinical Infectious Diseases 2009;48(12):1752‐9. [PUBMED: 19438397] - PubMed
Mateelli 2013 {unpublished data only}
-
- Mateelli A, Kouanda S, Saleri N, Ouedraogo G. Efficacy and safety of nevirapine‐ vs efavirenz based ART in TB/HIV patients in Burkina Faso: a clinical and pharmacokinetic study. 20th Conference on Retroviruses and Opportunistic Infections. 3‐16 March, 2013. Atlanta, USA, 2013:Abstract 47462.
Núñez 2002 {published data only}
-
- Núñez M, Soriano V, Martin‐Carbonero L, Barrios A, Barreiro P, Blanco F, et al. SENC (Spanish efavirenz vs. nevirapine comparison) trial: a randomized, open‐label study in HIV‐infected naïve individuals. HIV Clinical Trials 2002;3(3):186‐94. [PUBMED: 12032877] - PubMed
Sinha 2013 {published data only}
-
- Sinha S, Raghunandan P, Chandrashekhar R, Sharma SK, Kumar S, Dhooria S, et al. Nevirapine versus efavirenz‐based antiretroviral therapy regimens in antiretroviral‐naïve patients with HIV and tuberculosis infections in India: a pilot study. BMC Infectious Diseases 2013;13:482. [PUBMED: 24134449] - PMC - PubMed
Sow 2006 {published data only}
-
- Sow PG, Badiane M, Diallo PD, Lo I, Ndiaye B, Gaye AM. Efficacy and safety of lamivudine+zidovudine+efavirenz and lamivudine+zidovudine+nevirapine in treatment HIV1 infected patients. A cross study analysis [Abstract CDB0584]. XVI International AIDS Conference; 13‐18 August, 2006. Toronto, Canada, 2006.
Swaminathan 2011 {published data only}
-
- Swaminathan S, Padmapriyadarsini C, Venkatesan P, Narendran G, Ramesh Kumar S, Iliayas S, et al. Efficacy and safety of once‐daily nevirapine‐ or efavirenz‐based antiretroviral therapy in HIV‐associated tuberculosis: a randomized clinical trial. Clinical infectious disease 2011;53(7):716‐724. - PubMed
van den Berg‐Wolf 2008 {published data only}
-
- Berg‐Wolf M, Hullsiek KH, Peng G, Kozal MJ, Novak RM, Chen L, et al. Virologic, immunologic, clinical, safety, and resistance outcomes from a long‐term comparison of efavirenz‐based versus nevirapine‐based antiretroviral regimens as initial therapy in HIV‐1‐infected persons. HIV Clinical Trials 2008;9(5):324‐36. [PUBMED: 18977721] - PubMed
van Leth 2004 {published data only}
-
- Leth F, Phanuphak P, Ruxrungtham K, Baraldi E, Miller S, Gazzard B, et al. Comparison of first‐line antiretroviral therapy with regimens including nevirapine, efavirenz, or both drugs, plus stavudine and lamivudine: a randomised open‐label trial, the 2NN Study. Lancet 2004;363(9417):1253‐63. [PUBMED: 15094269] - PubMed
Wester 2010 {published data only}
References to studies excluded from this review
Antela 2004 {published data only}
-
- Antela A, Iribarren JA, Mahillo B, Santos I, Ribera E, Gutierrez C, et al. Final analysis of a prospective, randomized, open‐label, multicentre trial in naïve, HIV‐1‐infected patients, comparing ZDV/3TC vs d4T/ddI, plus efavirenz, nevirapine or indinavir/ritonavir (AMADEUS 01 study). International Conference on AIDS; 11‐16 July, 2004; Bangkok, Thailand. 2004; Vol. 15:Abstract no. B11920.
Bannister 2008 {published data only}
-
- Bannister WP, Ruiz L, Cozzi‐Lepri A, Mocroft A, Kirk O, Staszewski S, et al. Comparison of genotypic resistance profiles and virological response between patients starting nevirapine and efavirenz in EuroSIDA. AIDS (London, England) 2008;22(3):367‐76. [PUBMED: 18195563] - PubMed
Bonnet 2013b {published data only}
Brück 2008 {published data only}
-
- Brück S, Witte S, Brust J, Schuster D, Mosthaf F, Procaccianti M, et al. Hepatotoxicity in patients prescribed efavirenz or nevirapine. European Journal of Medical Research 2008;13(7):343‐8. - PubMed
de Beaudrap 2008 {published data only}
Han 2005 {published data only}
-
- Han XX, Zhang M, Cui WG, Liu BG, Wang Y, Zhang ZN, et al. Efficacy of anti‐HIV treatment and drug‐resistance mutations in some parts of China. Zhonghua Yi Xue Za Zhi 2005;85(11):760‐4. - PubMed
Hartmann 2005a {published data only}
-
- Hartmann M, Witte S, Brust J, Schuster D, Mosthaf F, Procaccianti M, et al. Comparison of efavirenz and nevirapine in HIV‐infected patients (NEEF Cohort). International Journal of STD & AIDS 2005;16(6):404‐9. [PUBMED: 15969773] - PubMed
Hartmann 2005b {published data only}
-
- Hartmann M, Brust J, Schuster D, Mosthaf F, Procaccianti M, Rump JA, et al. Rashes in HIV‐infected patients undergoing therapy with nevirapine or efavirenz [Arzneimittelexantheme bei Therapie der HIV‐Infektion mit Efavirenz und Nevirapin]. Der Hautarzt 2005;56(9):847‐53. - PubMed
He 2011 {published data only}
-
- He Y, Luo Y, Ding YL, Zheng YH, Li J, Huang J, et al. [Effect of highly active anti‐retroviral therapy on prevention of mother to child transmission of HIV and on infant growth and development]. Zhonghua Yu Fang Yi Xue Za Zhi [Chinese Journal of Preventive Medicine] 2011;45(10):912‐5. [PUBMED: 22321592] - PubMed
Lapphra 2008 {published data only}
-
- Lapphra K, Vanprapar N, Chearskul S, Phongsamart W, Chearskul P, Prasitsuebsai W, et al. Efficacy and tolerability of nevirapine‐ versus efavirenz‐containing regimens in HIV‐infected Thai children. International Journal of Infectious Diseases 2008;12(6):e33‐8. [PUBMED: 18573672] - PubMed
Manfredi 2004 {published data only}
-
- Manfredi R, Calza L, Chiodo F. Efavirenz versus nevirapine in current clinical practice: a prospective, open‐label observational study. Journal of Acquired Immune Deficiency Syndromes 2004;35(5):492‐502. - PubMed
Manfredi 2005 {published data only}
-
- Manfredi R, Calza L, Chiodo F. Prospective, open‐label comparative study of liver toxicity in an unselected population of HIV‐infected patients treated for the first time with efavirenz or nevirapine. HIV Clinical Trials 2005;6(6):302‐11. - PubMed
Manfredi 2006 {published data only}
-
- Manfredi R, Calza L. Nevirapine versus efavirenz in 742 patients: no link of liver toxicity with female sex, and a baseline CD4 cell count greater than 250 cells/microl. AIDS 2006;20(17):2233‐6. - PubMed
Mankhatitham 2011 {published data only}
-
- Mankhatitham W, Lueangniyomkul A, Manosuthi W. Hepatotoxicity in patients co‐infected with tuberculosis and HIV‐1 while receiving non‐nucleoside reverse transcriptase inhibitor‐based antiretroviral therapy and rifampicin‐containing anti‐tuberculosis regimen. Southeast Asian Journal of Tropical Medicine and Public Health 2011;42(3):651‐8. [PUBMED: 21706943] - PubMed
Mankhatitham 2012 {published data only}
-
- Mankhatitham W, Luaengniyomkul A, Manosuthi W. Lipid profile changes in Thai HIV and tuberculosis co‐infected patients receiving non‐nucleoside reverse transcriptase inhibitors‐based antiretroviral therapy. Chotmaihet thangphaet [Journal of the Medical Association of Thailand] 2012;95(2):163‐9. [PUBMED: 22435244] - PubMed
Manosuthi 2004 {published data only}
-
- Manosuthi W, Sungkanuparph S, Vibhagool A, Rattanasiri S, Thakkinstian A. Nevirapine‐ versus efavirenz‐based highly active antiretroviral therapy regimens in antiretroviral‐naïve patients with advanced HIV infection. HIV Medicine 2004;5(2):105‐9. - PubMed
Manosuthi 2009b {published data only}
-
- Manosuthi W, Mankatitham W, Lueangniyomkul A, Thongyen S, Prommool V, Likanonsakul S, et al. Serial monitoring of drug concentrations while on and off rifampicin between standard doses of nevirapine based and efavirenz‐based antiretroviral regimens. HIV Medicine 2009;10(Suppl 2):45‐221.
Musiime 2012 {published data only}
-
- Musiime V, Cook A, Kayiwa J, Zangata D, Nansubuga C, Arach B, et al. Differences in body circumferences, skin‐fold thicknesses and lipid profiles among HIV‐infected African children on and not on stavudine. Journal of the International AIDS Society 2012;15(Suppl 4):18290.
Nachega 2008 {published data only}
Negredo 2004 {published data only}
-
- Negredo E, Paredes R, Peraire J, Pedrol E, Côté H, Gel S, et al. Alteration of antiretroviral drug regimens for HIV infection. Efficacy, safety and tolerability at week 96 of the Swatch Study. Antiviral Therapy 2004;9(6):889‐93. - PubMed
Padmapriyadarsini 2013 {published data only}
-
- Padmapriyadarsini C, Bhavani PK, Tang A, Kumar H, Ponnuraja C, Narendran G, et al. Early changes in hepatic function among HIV‐tuberculosis patients treated with nevirapine or efavirenz along with rifampin‐based anti‐tuberculosis therapy. International Journal of Infectious Diseases 2013;17(12):e1154‐9. [PUBMED: 24120216] - PMC - PubMed
PENPACT 2011 {published data only}
-
- PENPACT‐1 (PENTA 9/PACTG 390) Study Team, Babiker A, Castro nee Green H, Compagnucci A, Fiscus S, Giaquinto C, et al. First‐line antiretroviral therapy with a protease inhibitor versus non‐nucleoside reverse transcriptase inhibitor and switch at higher versus low viral load in HIV‐infected children: an open‐label, randomised phase 2/3trial. Lancet Infect Diseases 2011;11(4):273‐83. - PMC - PubMed
Prendergast 2011 {published data only}
-
- Prendergast A, Bwakura‐Dangarembizi MF, Cook AD, Bakeera‐Kitaka S, Natukunda E, Nahirya Ntege P, et al. Hospitalization for severe malnutrition among HIV‐infected children starting antiretroviral therapy. AIDS 2011;25(7):951‐6. [PUBMED: 21487251] - PubMed
Puthanakit 2009a {published data only}
-
- Puthanakit T, Aurpibul L, Sirisanthana T, Sirisanthana V. Efficacy of non‐nucleoside reverse transcriptase inhibitor‐based highly active antiretroviral therapy in Thai HIV‐infected children aged two years or less. Pediatric Infectious Disease Journal 2009;28(3):246‐8. [PUBMED: 19165130] - PubMed
Puthanakit 2009b {published data only}
-
- Puthanakit T, Kerr SJ, Ananworanich J, Bunupuradah T, Boonrak P, Sirisanthana V. Pattern and predictors of immunologic recovery in human immunodeficiency virus‐infected children receiving non‐nucleoside reverse transcriptase inhibitor‐based highly active antiretroviral therapy. Pediatric Infectious Disease Journal 2009;28(6):488‐92. - PubMed
Swaminathan 2009 {published data only}
-
- Swaminathan S, Padmapriyadarsini C, Venkatesan P, Narendran G, Ramesh Kumar S, Iliayas S, et al. Once‐daily nevirapine vs efavirenz in the treatment of HIV‐infected patients with TB: a randomized clinical trial. 16th conference on retroviruses and opportunistic infections. Montreal, Canada, February 8‐11, 2009.. 2009. [PUBMED: 21890776] - PubMed
Additional references
Beck 2008
-
- Beck EJ, Mandalia S, Youle M, Brettle R, Fisher M, Gompels M, et al. Treatment outcome and cost‐effectiveness of different highly active antiretroviral therapy regimens in the UK (1996‐2002). International Journal of STD & AIDS 2008;19(5):297‐304. - PubMed
BHIVA 2001
-
- BHIVA Writing Committee, BHIVA Executive Committee. Guidelines for the treatment of HIV‐infected adults with antiretroviral therapy. HIV Medicine 2001;2(4):276‐313. - PubMed
Chou 2006
-
- Chou R, Rongwei F, Huffman LH, Korthuis PT. Initial highly‐active antiretroviral therapy with a protease inhibitor versus a non‐nucleoside reverse transcriptase inhibitor: discrepancies between direct and indirect meta‐analyses. Lancet 2006;368(9546):1503‐15. - PubMed
Cooper 2007
-
- Cooper CL, Heeswijk RPG. Once‐daily nevirapine dosing: a pharmacokinetics, efficacy and safety review. HIV Medicine 2007;8(1):1‐7. - PubMed
Deeks 2001
-
- Deeks SG. International perspectives on antiretroviral resistance. Nonnucleoside reverse transcriptase inhibitor resistance. Journal of Acquired Immune Deficiency Syndromes 2001;26 Suppl 1:S25‐33. - PubMed
DHHS 2001a
-
- Department of Health and Human Sciences. AIDSinfo Drug Database. https://aidsinfo.nih.gov/drugs/116/nevirapine/0/professional (accessed 10 November 2016).
DHHS 2001b
-
- Department of Health and Human Sciences. AIDSinfo Drug Database. https://aidsinfo.nih.gov/drugs/269/efavirenz/0/professional (accessed 10 November 2016).
Dybul 2002
-
- Dybul M, Fauci AS, Bartlett JG, Kaplan JE, Pau AK, Panel on Clinical Practices for the Treatment of HIV. Guidelines for using antiretroviral agents among HIV‐infected adults and adolescents. Recommendations of the Panel on Clinical Practices for Treatment of HIV. Morbidity and Mortality Weekly Report 2002;51(RR‐7):1‐55. - PubMed
Egger 1997
Gilks 2006
-
- Gilks CF, Crowley S, Ekpini R, Gove S, Perriens J, Souteyrand Y, et al. The WHO public‐health approach to antiretroviral treatment against HIV in resource‐limited settings. Lancet 2006;368(9534):505‐10. - PubMed
GRADEpro 2014 [Computer program]
-
- GRADE Working Group, McMaster University. GRADEpro GDT. Version (assessed 03 November 2016). Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Guyatt 2008
Higgins 2008
-
- Higgins JPT, Green S, editor(s). In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley & Sons, 2008.
Hogg 1997
-
- Hogg RS, O'Shaughnessy MV, Gataric N, Yip B, Craib K, Schechter MT, et al. Decline in deaths from AIDS due to new antiretrovirals. Lancet 1997;349(9061):1294. - PubMed
Jiang 2014
-
- Jiang HY, Zhang MN, Chen HJ, Yang Y, Deng M, Ruan B. Nevirapine versus efavirenz for patients co‐infected with HIV and tuberculosis: a systematic review and meta‐analysis. International Journal of Infectious Diseases 2014;25:130‐5. [PUBMED: 24911886] - PubMed
Lau 2007
-
- Lau B, Gange S, Kirk G, Mehta S, Merriman B, Moore R. Predictive value of plasma HIV RNA levels for rate of CD4 decline and clinical disease progression. 14th Conference on Retroviruses and Opportunistic Infections (CROI); 25‐28 February, 2007; Los Angeles, California. 2007, issue Abstract 140 (oral).
Mellors 2007
-
- Mellors J, Margolick J, Phair J, Rinaldo C, Detels R, Jaconson L, et al. Comparison of plasma HIV‐1 RNA, CD4 cell count, and CD38 expression on CD8 T cells as predictors of progression to AIDS and CD4 cell decline among untreated participants in the multicenter AIDS cohort study. 14th Conference on Retroviruses and Opportunistic Infections; 25‐28 February, 2007; Los Angeles, California. 2007.
Mocroft 1998
-
- Mocroft A, Vella S, Benfield TL, Chiesi A, Miller V, Gargalianos P, et al. Changing patterns of mortality across Europe in patients infected with HIV‐1. EuroSIDA Study Group. Lancet 1998;352(9142):1725‐30. - PubMed
Moyle 2000
-
- Moyle GJ. Considerations in the choice of protease inhibitor‐sparing regimens in initial therapy for HIV‐1 infection. Current Opinion in Infectious Diseases 2000;13(1):19‐25. - PubMed
NIAID/NIH 2004
-
- Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health. Table for Grading the Severity of Adult and Pediatric Adverse Events. Bethesda, Maryland: National Institutes of Health, 2004.
Pillay 2013
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Shubber 2013
-
- Shubber Z, Calmy A, Andrieux‐Meyer I, Vitoria M, Renaud‐Théry F, Shaffer N, et al. Adverse events associated with nevirapine and efavirenz‐based first‐line antiretroviral therapy: a systematic review and meta‐analysis. AIDS (London, England) 2013;27(9):1403‐12. [PUBMED: 23343913] - PubMed
Siegfried 2006
UNAIDS 2016
-
- UNAIDS. AIDS by the numbers 2015. http://www.unaids.org/sites/default/files/media_asset/AIDS‐by‐the‐number... (accessed 03 November 2016).
Veldkamp 2001
-
- Veldkamp AI, Weverling GJ, Lange JM, Montaner JS, Reiss P, Cooper DA, et al. High exposure to nevirapine in plasma is associated with an improved virological response in HIV‐1‐infected individuals. AIDS 2001;15(9):1089‐95. - PubMed
WHO 2002
-
- World Health Organization. Scaling up antiretroviral therapy in low in resource‐limited settings. Guidelines for a public health approach. June 2002. http://www.who.int/hiv/pub/prev_care/en/ScalingUp_E.pdf (accessed 03 November 2016).
WHO 2006
-
- World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. 2006 revision. http://whqlibdoc.who.int/publications/2006/9789241594677_eng.pdf (accessed 25 September 2008). - PubMed
WHO 2009
-
- World Health Organization. Rapid advice: antiretroviral therapy for HIV infection in adults and adolescents. November 2009. http://www.who.int/hiv/pub/arv/rapid_advice_art.pdf (accessed 03 November 2016).
WHO 2014
-
- World Health Organization. Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations. July 2014. http://www.who.int/hiv/pub/guidelines/keypopulations/en/ (accessed 09 September 2016). - PubMed
WHO 2015a
-
- World Health Organization. WHO Model List of Essential Medicines. 19th List. http://www.who.int/medicines/publications/essentialmedicines/EML_2015_FI... (accessed 10 September 2008).
WHO 2015b
-
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. Second edition. http://www.who.int/hiv/pub/arv/arv‐2016/en/ (accessed 03 November 2016). - PubMed
References to other published versions of this review
Mbuagbaw 2009
Mbuagbaw 2010
-
- Mbuagbaw LC, Irlam JH, Spaulding A, Rutherford GW, Siegfried N. Efavirenz or nevirapine in three‐drug combination therapy with two nucleoside‐reverse transcriptase inhibitors for initial treatment of HIV infection in antiretroviral‐naïve individuals. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD004246.pub3] - DOI - PubMed
Neuwelt MD 2002
-
- Neuwelt MD. Efavirenz versus nevirapine as a non‐nucleoside reverse transcriptase inhibitor in initial combination antiretroviral therapy for HIV infection. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD004246] - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

