Regulator of Cell Cycle (RGCC) Expression During the Progression of Alzheimer's Disease
- PMID: 27938491
- PMCID: PMC5661221
- DOI: 10.3727/096368916X694184
Regulator of Cell Cycle (RGCC) Expression During the Progression of Alzheimer's Disease
Abstract
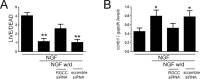
Unscheduled cell cycle reentry of postmitotic neurons has been described in cases of mild cognitive impairment (MCI) and Alzheimer's disease (AD) and may form a basis for selective neuronal vulnerability during disease progression. In this regard, the multifunctional protein regulator of cell cycle (RGCC) has been implicated in driving G1/S and G2/M phase transitions through its interactions with cdc/cyclin-dependent kinase 1 (cdk1) and is induced by p53, which mediates apoptosis in neurons. We tested whether RGCC levels were dysregulated in frontal cortex samples obtained postmortem from subjects who died with a clinical diagnosis of no cognitive impairment (NCI), MCI, or AD. RGCC mRNA and protein levels were upregulated by ∼50%-60% in MCI and AD compared to NCI, and RGCC protein levels were associated with poorer antemortem global cognitive performance in the subjects examined. To test whether RGCC might regulate neuronal cell cycle reentry and apoptosis, we differentiated neuronotypic PC12 cultures with nerve growth factor (NGF) followed by NGF withdrawal to induce abortive cell cycle activation and cell death. Experimental reduction of RGCC levels increased cell survival and reduced levels of the cdk1 target cyclin B1. RGCC may be a candidate cell cycle target for neuroprotection during the onset of AD.
Figures
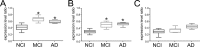
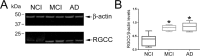
Similar articles
-
Selective decline of neurotrophin and neurotrophin receptor genes within CA1 pyramidal neurons and hippocampus proper: Correlation with cognitive performance and neuropathology in mild cognitive impairment and Alzheimer's disease.Hippocampus. 2019 May;29(5):422-439. doi: 10.1002/hipo.22802. Epub 2017 Sep 27. Hippocampus. 2019. PMID: 28888073 Free PMC article.
-
Frontal cortex chitinase and pentraxin neuroinflammatory alterations during the progression of Alzheimer's disease.J Neuroinflammation. 2020 Feb 17;17(1):58. doi: 10.1186/s12974-020-1723-x. J Neuroinflammation. 2020. PMID: 32066474 Free PMC article.
-
Elevated expression of a regulator of the G2/M phase of the cell cycle, neuronal CIP-1-associated regulator of cyclin B, in Alzheimer's disease.J Neurosci Res. 2004 Mar 1;75(5):698-703. doi: 10.1002/jnr.20028. J Neurosci Res. 2004. PMID: 14991845
-
Emerging Roles of Inhibitor of Differentiation-1 in Alzheimer's Disease: Cell Cycle Reentry and Beyond.Cells. 2020 Jul 21;9(7):1746. doi: 10.3390/cells9071746. Cells. 2020. PMID: 32708313 Free PMC article. Review.
-
Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
Cited by
-
Unraveling the Mechanism of Immunity and Inflammation Related to Molecular Signatures Crosstalk Among Obesity, T2D, and AD: Insights From Bioinformatics Approaches.Bioinform Biol Insights. 2023 Apr 25;17:11779322231167977. doi: 10.1177/11779322231167977. eCollection 2023. Bioinform Biol Insights. 2023. PMID: 37124128 Free PMC article.
-
Comprehensive analysis of core genes and key pathways in Parkinson's disease.Am J Transl Res. 2020 Sep 15;12(9):5630-5639. eCollection 2020. Am J Transl Res. 2020. PMID: 33042444 Free PMC article.
-
Unravelling Mechanisms of Doxorubicin-Induced Toxicity in 3D Human Intestinal Organoids.Int J Mol Sci. 2022 Jan 24;23(3):1286. doi: 10.3390/ijms23031286. Int J Mol Sci. 2022. PMID: 35163210 Free PMC article.
-
Systematic Analysis of Differential Expression Profile in Rheumatoid Arthritis Chondrocytes Using Next-Generation Sequencing and Bioinformatics Approaches.Int J Med Sci. 2018 Jul 13;15(11):1129-1142. doi: 10.7150/ijms.27056. eCollection 2018. Int J Med Sci. 2018. PMID: 30123050 Free PMC article.
-
Intracerebral matrix metalloproteinase 9 in fatal diabetic ketoacidosis.Exp Mol Pathol. 2019 Jun;108:97-104. doi: 10.1016/j.yexmp.2019.04.008. Epub 2019 Apr 13. Exp Mol Pathol. 2019. PMID: 30986397 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

