MERS-CoV spike protein: a key target for antivirals
- PMID: 27936982
- PMCID: PMC5457961
- DOI: 10.1080/14728222.2017.1271415
MERS-CoV spike protein: a key target for antivirals
Abstract
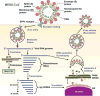
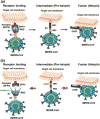
The continual Middle East respiratory syndrome (MERS) threat highlights the importance of developing effective antiviral therapeutics to prevent and treat MERS coronavirus (MERS-CoV) infection. A surface spike (S) protein guides MERS-CoV entry into host cells by binding to cellular receptor dipeptidyl peptidase-4 (DPP4), followed by fusion between virus and host cell membranes. MERS-CoV S protein represents a key target for developing therapeutics to block viral entry and inhibit membrane fusion. Areas covered: This review illustrates MERS-CoV S protein's structure and function, particularly S1 receptor-binding domain (RBD) and S2 heptad repeat 1 (HR1) as therapeutic targets, and summarizes current advancement on developing anti-MERS-CoV therapeutics, focusing on neutralizing monoclonal antibodies (mAbs) and antiviral peptides. Expert opinion: No anti-MERS-CoV therapeutic is approved for human use. Several S-targeting neutralizing mAbs and peptides have demonstrated efficacy against MERS-CoV infection, providing feasibility for development. Generally, human neutralizing mAbs targeting RBD are more potent than those targeting other regions of S protein. However, emergence of escape mutant viruses and mAb's limitations make it necessary for combining neutralizing mAbs recognizing different neutralizing epitopes and engineering them with improved efficacy and reduced cost. Optimization of the peptide sequences is expected to produce next-generation anti-MERS-CoV peptides with improved potency.
Keywords: MERS; MERS-CoV; membrane fusion; monoclonal antibodies; peptides; receptor-binding domain; spike protein; therapeutics.
Conflict of interest statement
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Figures

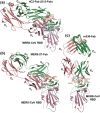
Similar articles
-
Recombinant Receptor-Binding Domains of Multiple Middle East Respiratory Syndrome Coronaviruses (MERS-CoVs) Induce Cross-Neutralizing Antibodies against Divergent Human and Camel MERS-CoVs and Antibody Escape Mutants.J Virol. 2016 Dec 16;91(1):e01651-16. doi: 10.1128/JVI.01651-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795425 Free PMC article.
-
[Development of peptidic MERS-CoV entry inhibitors].Yao Xue Xue Bao. 2015 Dec;50(12):1513-9. Yao Xue Xue Bao. 2015. PMID: 27169270 Review. Chinese.
-
A Novel Nanobody Targeting Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Receptor-Binding Domain Has Potent Cross-Neutralizing Activity and Protective Efficacy against MERS-CoV.J Virol. 2018 Aug 29;92(18):e00837-18. doi: 10.1128/JVI.00837-18. Print 2018 Sep 15. J Virol. 2018. PMID: 29950421 Free PMC article.
-
Identification of a Novel Inhibitor against Middle East Respiratory Syndrome Coronavirus.Viruses. 2017 Sep 14;9(9):255. doi: 10.3390/v9090255. Viruses. 2017. PMID: 28906430 Free PMC article.
-
Neutralizing Monoclonal Antibodies as Promising Therapeutics against Middle East Respiratory Syndrome Coronavirus Infection.Viruses. 2018 Nov 30;10(12):680. doi: 10.3390/v10120680. Viruses. 2018. PMID: 30513619 Free PMC article. Review.
Cited by
-
The Inclusive Review on SARS-CoV-2 Biology, Epidemiology, Diagnosis, and Potential Management Options.Curr Microbiol. 2021 Apr;78(4):1099-1114. doi: 10.1007/s00284-021-02396-x. Epub 2021 Feb 27. Curr Microbiol. 2021. PMID: 33638671 Free PMC article. Review.
-
SARS-CoV-2 and Coronavirus Disease Mitigation: Treatment Options, Vaccinations and Variants.Pathogens. 2022 Feb 20;11(2):275. doi: 10.3390/pathogens11020275. Pathogens. 2022. PMID: 35215217 Free PMC article. Review.
-
Superior immune responses induced by intranasal immunization with recombinant adenovirus-based vaccine expressing full-length Spike protein of Middle East respiratory syndrome coronavirus.PLoS One. 2019 Jul 22;14(7):e0220196. doi: 10.1371/journal.pone.0220196. eCollection 2019. PLoS One. 2019. PMID: 31329652 Free PMC article.
-
Insight into free energy and dynamic cross-correlations of residue for binding affinity of antibody and receptor binding domain SARS-CoV-2.Heliyon. 2023 Jan;9(1):e12667. doi: 10.1016/j.heliyon.2022.e12667. Epub 2023 Jan 3. Heliyon. 2023. PMID: 36618128 Free PMC article.
-
Current understanding of middle east respiratory syndrome coronavirus infection in human and animal models.J Thorac Dis. 2018 Jul;10(Suppl 19):S2260-S2271. doi: 10.21037/jtd.2018.03.80. J Thorac Dis. 2018. PMID: 30116605 Free PMC article. Review.
References
-
- Zaki AM, Van BS, Bestebroer TM, et al. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820. This is a paper describing the first isolation of Middle East respiratory syndrome coronavirus (MERS-CoV) in humans. - PubMed
-
- Bermingham A, Chand MA, Brown CS, et al. Severe respiratory illness caused by a novel coronavirus, in a patient transferred to the United Kingdom from the Middle East, September 2012. Euro Surveill. 2012;17(40):20290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
