Hyperhomocysteinemia in ApoE-/- Mice Leads to Overexpression of Enhancer of Zeste Homolog 2 via miR-92a Regulation
- PMID: 27936205
- PMCID: PMC5147974
- DOI: 10.1371/journal.pone.0167744
Hyperhomocysteinemia in ApoE-/- Mice Leads to Overexpression of Enhancer of Zeste Homolog 2 via miR-92a Regulation
Erratum in
-
Correction: Hyperhomocysteinemia in ApoE-/- Mice Leads to Overexpression of Enhancer of Zeste Homolog 2 via miR-92a Regulation.PLoS One. 2020 Oct 12;15(10):e0240762. doi: 10.1371/journal.pone.0240762. eCollection 2020. PLoS One. 2020. PMID: 33045026 Free PMC article.
Abstract
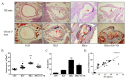
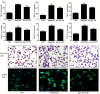
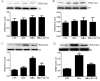
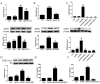
Hyperhomocysteinemia (HHcy) is an independent risk factor for cardiovascular diseases, such as atherosclerosis. HHcy promotes atherogenesis by modifying the histone methylation patterns and miRNA regulation. In this study, we investigated the effects of homocysteine (Hcy) on the expression of enhancer of zeste homolog 2 (EZH2), and tested our hypothesis that Hcy-induced atherosclerosis is mediated by increased EZH2 expression, which is regulated by miR-92a. The levels of EZH2 and H3K27me3 were increased in the aorta of ApoE-/- mice fed a high-methionine diet for 16 weeks, whereas miR-92a expression was decreased. Over-expression of EZH2 increased H3K27me3 level and the accumulation of total cholesterol and triglycerides in the foam cells. Furthermore, upregulation of miR-92a reduced EZH2 expression in the foam cells. These data suggested that EZH2 plays a key role in Hcy-mediated lipid metabolism disorders, and that miR-92a may be a novel therapeutic target in Hcy-related atherosclerosis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
miR-34a Targets HDAC1-Regulated H3K9 Acetylation on Lipid Accumulation Induced by Homocysteine in Foam Cells.J Cell Biochem. 2017 Dec;118(12):4617-4627. doi: 10.1002/jcb.26126. Epub 2017 Jun 12. J Cell Biochem. 2017. PMID: 28485501
-
Enhancer of zeste homolog 2 participates in the process of atherosclerosis by modulating microRNA-139-5p methylation and signal transducer and activator of transcription 1 expression.IUBMB Life. 2021 Jan;73(1):238-251. doi: 10.1002/iub.2423. Epub 2020 Dec 17. IUBMB Life. 2021. PMID: 33331071
-
Histone Methyltransferase Enhancer of Zeste Homolog 2-Mediated ABCA1 Promoter DNA Methylation Contributes to the Progression of Atherosclerosis.PLoS One. 2016 Jun 13;11(6):e0157265. doi: 10.1371/journal.pone.0157265. eCollection 2016. PLoS One. 2016. PMID: 27295295 Free PMC article.
-
Epigenetic Regulation by microRNAs in Hyperhomocysteinemia-Accelerated Atherosclerosis.Int J Mol Sci. 2022 Oct 18;23(20):12452. doi: 10.3390/ijms232012452. Int J Mol Sci. 2022. PMID: 36293305 Free PMC article. Review.
-
The pathophysiological hypothesis of homocysteine thiolactone-mediated vascular disease.J Physiol Pharmacol. 2008 Dec;59 Suppl 9:155-67. J Physiol Pharmacol. 2008. PMID: 19261978 Review.
Cited by
-
Correction: Hyperhomocysteinemia in ApoE-/- Mice Leads to Overexpression of Enhancer of Zeste Homolog 2 via miR-92a Regulation.PLoS One. 2020 Oct 12;15(10):e0240762. doi: 10.1371/journal.pone.0240762. eCollection 2020. PLoS One. 2020. PMID: 33045026 Free PMC article.
-
Flow-dependent epigenetic regulation of IGFBP5 expression by H3K27me3 contributes to endothelial anti-inflammatory effects.Theranostics. 2018 Apr 30;8(11):3007-3021. doi: 10.7150/thno.21966. eCollection 2018. Theranostics. 2018. PMID: 29896299 Free PMC article.
-
Repressive H3K27me3 drives hyperglycemia-induced oxidative and inflammatory transcriptional programs in human endothelium.Cardiovasc Diabetol. 2024 Apr 5;23(1):122. doi: 10.1186/s12933-024-02196-0. Cardiovasc Diabetol. 2024. PMID: 38580969 Free PMC article.
-
Phenethyl Isothiocyanate Protects against High Fat/Cholesterol Diet-Induced Obesity and Atherosclerosis in C57BL/6 Mice.Nutrients. 2020 Nov 27;12(12):3657. doi: 10.3390/nu12123657. Nutrients. 2020. PMID: 33261070 Free PMC article.
-
Knockdown of GAS5 Inhibits Atherosclerosis Progression via Reducing EZH2-Mediated ABCA1 Transcription in ApoE-/- Mice.Mol Ther Nucleic Acids. 2020 Mar 6;19:84-96. doi: 10.1016/j.omtn.2019.10.034. Epub 2019 Nov 12. Mol Ther Nucleic Acids. 2020. PMID: 31830648 Free PMC article.
References
-
- Araldi E, Chamorro-Jorganes A, van Solingen C, Fernandez-Hernando C, Suarez Y. Therapeutic Potential of Modulating microRNAs in Atherosclerotic Vascular Disease. Current vascular pharmacology. 2015; 13:291–304 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

