Genetic, immunological, and clinical features of patients with bacterial and fungal infections due to inherited IL-17RA deficiency
- PMID: 27930337
- PMCID: PMC5187691
- DOI: 10.1073/pnas.1618300114
Genetic, immunological, and clinical features of patients with bacterial and fungal infections due to inherited IL-17RA deficiency
Abstract
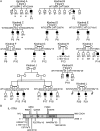
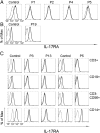
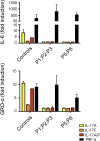
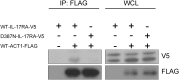
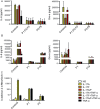
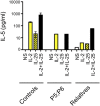
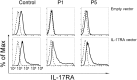
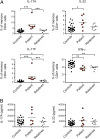
Chronic mucocutaneous candidiasis (CMC) is defined as recurrent or persistent infection of the skin, nails, and/or mucosae with commensal Candida species. The first genetic etiology of isolated CMC-autosomal recessive (AR) IL-17 receptor A (IL-17RA) deficiency-was reported in 2011, in a single patient. We report here 21 patients with complete AR IL-17RA deficiency, including this first patient. Each patient is homozygous for 1 of 12 different IL-17RA alleles, 8 of which create a premature stop codon upstream from the transmembrane domain and have been predicted and/or shown to prevent expression of the receptor on the surface of circulating leukocytes and dermal fibroblasts. Three other mutant alleles create a premature stop codon downstream from the transmembrane domain, one of which encodes a surface-expressed receptor. Finally, the only known missense allele (p.D387N) also encodes a surface-expressed receptor. All of the alleles tested abolish cellular responses to IL-17A and -17F homodimers and heterodimers in fibroblasts and to IL-17E/IL-25 in leukocytes. The patients are currently aged from 2 to 35 y and originate from 12 unrelated kindreds. All had their first CMC episode by 6 mo of age. Fourteen patients presented various forms of staphylococcal skin disease. Eight were also prone to various bacterial infections of the respiratory tract. Human IL-17RA is, thus, essential for mucocutaneous immunity to Candida and Staphylococcus, but otherwise largely redundant. A diagnosis of AR IL-17RA deficiency should be considered in children or adults with CMC, cutaneous staphylococcal disease, or both, even if IL-17RA is detected on the cell surface.
Keywords: candidiasis; genetics; immunodeficiency.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity.Science. 2011 Apr 1;332(6025):65-8. doi: 10.1126/science.1200439. Epub 2011 Feb 24. Science. 2011. PMID: 21350122 Free PMC article.
-
Inherited IL-17RC deficiency in patients with chronic mucocutaneous candidiasis.J Exp Med. 2015 May 4;212(5):619-31. doi: 10.1084/jem.20141065. Epub 2015 Apr 27. J Exp Med. 2015. PMID: 25918342 Free PMC article.
-
A family with interleukin-17 receptor A deficiency: a case report and review of the literature.Turk J Pediatr. 2023;65(1):135-143. doi: 10.24953/turkjped.2022.40. Turk J Pediatr. 2023. PMID: 36866994 Review.
-
Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis.Curr Opin Allergy Clin Immunol. 2012 Dec;12(6):616-22. doi: 10.1097/ACI.0b013e328358cc0b. Curr Opin Allergy Clin Immunol. 2012. PMID: 23026768 Free PMC article. Review.
-
An ACT1 mutation selectively abolishes interleukin-17 responses in humans with chronic mucocutaneous candidiasis.Immunity. 2013 Oct 17;39(4):676-86. doi: 10.1016/j.immuni.2013.09.002. Epub 2013 Oct 10. Immunity. 2013. PMID: 24120361 Free PMC article.
Cited by
-
Genetic causes of primary immunodeficiency in the Jordanian population.Biomed Rep. 2024 Aug 30;21(5):160. doi: 10.3892/br.2024.1848. eCollection 2024 Nov. Biomed Rep. 2024. PMID: 39268404 Free PMC article.
-
Retrospective identification of the first cord blood-transplanted severe aplastic anemia in a STAT1-associated chronic mucocutaneous candidiasis family: case report, review of literature and pathophysiologic background.Front Immunol. 2024 Jul 24;15:1430938. doi: 10.3389/fimmu.2024.1430938. eCollection 2024. Front Immunol. 2024. PMID: 39114664 Free PMC article. Review.
-
Inborn errors of immunity with susceptibility to S. aureus infections.Front Pediatr. 2024 Apr 24;12:1389650. doi: 10.3389/fped.2024.1389650. eCollection 2024. Front Pediatr. 2024. PMID: 38720948 Free PMC article. Review.
-
Friend or Foe - Tc17 cell generation and current evidence for their importance in human disease.Discov Immunol. 2023 Jul 20;2(1):kyad010. doi: 10.1093/discim/kyad010. eCollection 2023. Discov Immunol. 2023. PMID: 38567057 Free PMC article. Review.
-
The IL-17 pathway as a target in giant cell arteritis.Front Immunol. 2024 Jan 17;14:1199059. doi: 10.3389/fimmu.2023.1199059. eCollection 2023. Front Immunol. 2024. PMID: 38299156 Free PMC article. Review.
References
-
- Kisand K, Peterson P. Autoimmune polyendocrinopathy candidiasis ectodermal dystrophy. J Clin Immunol. 2015;35(5):463–478. - PubMed
-
- Chilgren RA, Quie PG, Meuwissen HJ, Hong R. Chronic mucocutaneous candidiasis, deficiency of delayed hypersensitivity, and selective local antibody defect. Lancet. 1967;2(7518):688–693. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

