Withania somnifera Reverses Transactive Response DNA Binding Protein 43 Proteinopathy in a Mouse Model of Amyotrophic Lateral Sclerosis/Frontotemporal Lobar Degeneration
- PMID: 27928708
- PMCID: PMC5398980
- DOI: 10.1007/s13311-016-0499-2
Withania somnifera Reverses Transactive Response DNA Binding Protein 43 Proteinopathy in a Mouse Model of Amyotrophic Lateral Sclerosis/Frontotemporal Lobar Degeneration
Abstract
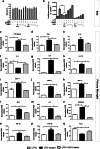
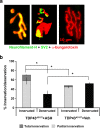
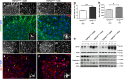
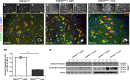
Abnormal cytoplasmic mislocalization of transactive response DNA binding protein 43 (TARDBP or TDP-43) in degenerating neurons is a hallmark of amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration with ubiquitin-positive inclusions (FTLD-U). Our previous work suggested that nuclear factor kappa B (NF-κB) may constitute a therapeutic target for TDP-43-mediated disease. Here, we investigated the effects of root extract of Withania somnifera (Ashwagandha), an herbal medicine with anti-inflammatory properties, in transgenic mice expressing a genomic fragment encoding human TDP-43A315T mutant. Ashwagandha extract was administered orally to hTDP-43A315T mice for a period of 8 weeks starting at 64 and 48 weeks of age for males and females, respectively. The treatment of hTDP-43A315T mice ameliorated their motor performance on rotarod test and cognitive function assessed by the passive avoidance test. Microscopy examination of tissue samples revealed that Ashwagandha treatment of hTDP-43A315T mice improved innervation at neuromuscular junctions, attenuated neuroinflammation, and reduced NF-κB activation. Remarkably, Ashwagandha treatment reversed the cytoplasmic mislocalization of hTDP-43 in spinal motor neurons and in brain cortical neurons of hTDP-43A315T mice and it reduced hTDP-43 aggregation. In vitro evidence is presented that the neuronal rescue of TDP-43 mislocalization may be due to the indirect effect of factors released from microglial cells exposed to Ashwagandha. These results suggest that Ashwagandha and its constituents might represent promising therapeutics for TDP-43 proteinopathies.
Keywords: Amyotrophic lateral sclerosis; NF-κB.; TDP-43; Withania somnifera.
Figures


Similar articles
-
Pathological hallmarks of amyotrophic lateral sclerosis/frontotemporal lobar degeneration in transgenic mice produced with TDP-43 genomic fragments.Brain. 2011 Sep;134(Pt 9):2610-26. doi: 10.1093/brain/awr159. Epub 2011 Jul 13. Brain. 2011. PMID: 21752789
-
Short-term suppression of A315T mutant human TDP-43 expression improves functional deficits in a novel inducible transgenic mouse model of FTLD-TDP and ALS.Acta Neuropathol. 2015 Nov;130(5):661-78. doi: 10.1007/s00401-015-1486-0. Epub 2015 Oct 5. Acta Neuropathol. 2015. PMID: 26437864
-
Withaferin-A Treatment Alleviates TAR DNA-Binding Protein-43 Pathology and Improves Cognitive Function in a Mouse Model of FTLD.Neurotherapeutics. 2021 Jan;18(1):286-296. doi: 10.1007/s13311-020-00952-0. Epub 2020 Oct 19. Neurotherapeutics. 2021. PMID: 33078279 Free PMC article.
-
Amyotrophic lateral sclerosis and frontotemporal lobar degeneration: a spectrum of TDP-43 proteinopathies.Neuropathology. 2010 Apr;30(2):103-12. doi: 10.1111/j.1440-1789.2009.01091.x. Epub 2010 Jan 25. Neuropathology. 2010. PMID: 20102519 Free PMC article. Review.
-
TDP-43 proteinopathy in frontotemporal lobar degeneration and amyotrophic lateral sclerosis: From pathomechanisms to therapeutic strategies.Ageing Res Rev. 2024 Sep;100:102441. doi: 10.1016/j.arr.2024.102441. Epub 2024 Jul 27. Ageing Res Rev. 2024. PMID: 39069095 Review.
Cited by
-
Anti-Neuroinflammatory Effects of Adaptogens: A Mini-Review.Molecules. 2024 Feb 15;29(4):866. doi: 10.3390/molecules29040866. Molecules. 2024. PMID: 38398618 Free PMC article. Review.
-
Neuroprotection: Targeting Multiple Pathways by Naturally Occurring Phytochemicals.Biomedicines. 2020 Aug 12;8(8):284. doi: 10.3390/biomedicines8080284. Biomedicines. 2020. PMID: 32806490 Free PMC article. Review.
-
Mitigation of ALS Pathology by Neuron-Specific Inhibition of Nuclear Factor Kappa B Signaling.J Neurosci. 2020 Jun 24;40(26):5137-5154. doi: 10.1523/JNEUROSCI.0536-20.2020. Epub 2020 May 26. J Neurosci. 2020. PMID: 32457070 Free PMC article.
-
Neuronal dysfunction caused by FUSR521G promotes ALS-associated phenotypes that are attenuated by NF-κB inhibition.Acta Neuropathol Commun. 2023 Nov 16;11(1):182. doi: 10.1186/s40478-023-01671-1. Acta Neuropathol Commun. 2023. PMID: 37974279 Free PMC article.
-
Standardized phytotherapic extracts rescue anomalous locomotion and electrophysiological responses of TDP-43 Drosophila melanogaster model of ALS.Sci Rep. 2018 Oct 30;8(1):16002. doi: 10.1038/s41598-018-34452-1. Sci Rep. 2018. PMID: 30375462 Free PMC article.
References
-
- Benajiba L, Le Ber I, Camuzat A, et al. TARDBP mutations in motoneuron disease with frontotemporal lobar degeneration. Ann Neurol. 2009;65:470–473. - PubMed
-
- Ayala YM, Zago P, D'Ambrogio A, et al. Structural determinants of the cellular localization and shuttling of TDP-43. J Cell Sci. 2008;121:3778–3785. - PubMed
-
- Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

